Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis
- PMID: 28774999
- PMCID: PMC5698077
- DOI: 10.1681/ASN.2017030295
Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis
Abstract
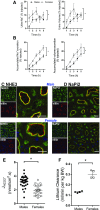
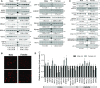
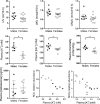
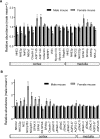
Compared with males, females have lower BP before age 60, blunted hypertensive response to angiotensin II, and a leftward shift in pressure natriuresis. This study tested the concept that this female advantage associates with a distinct sexual dimorphic pattern of transporters along the nephron. We applied quantitative immunoblotting to generate profiles of transporters, channels, claudins, and selected regulators in both sexes and assessed the physiologic consequences of the differences. In rats, females excreted a saline load more rapidly than males did. Compared with the proximal tubule of males, the proximal tubule of females had greater phosphorylation of Na+/H+ exchanger isoform 3 (NHE3), distribution of NHE3 at the base of the microvilli, and less abundant expression of Na+/Pi cotransporter 2, claudin-2, and aquaporin 1. These changes associated with less bicarbonate reabsorption and higher lithium clearance in females. The distal nephrons of females had a higher abundance of total and phosphorylated Na+/Cl- cotransporter (NCC), claudin-7, and cleaved forms of epithelial Na+ channel (ENaC) α and γ subunits, which associated with a lower baseline plasma K+ concentration. A K+-rich meal increased the urinary K+ concentration and decreased the level of renal phosphorylated NCC in females. Notably, we observed similar abundance profiles in female versus male C57BL/6 mice. These results define sexual dimorphic phenotypes along the nephron and suggest that lower proximal reabsorption in female rats expedites excretion of a saline load and enhances NCC and ENaC abundance and activation, which may facilitate K+ secretion and set plasma K+ at a lower level.
Keywords: Na transport; distal tubule; gender difference; potassium; proximal tubule.
Copyright © 2017 by the American Society of Nephrology.
Figures



Comment in
-
Renal physiology: The sexually dimorphic kidney.Nat Rev Nephrol. 2017 Oct;13(10):596. doi: 10.1038/nrneph.2017.122. Epub 2017 Aug 21. Nat Rev Nephrol. 2017. PMID: 28824173 No abstract available.
References
-
- Miller VM, Reckelhoff JF: Sex as a biological variable: Now What?! Physiology (Bethesda) 31: 78–80, 2016 - PubMed
-
- Maynard SE, Karumanchi SA, Thadhani R: Hypertension and Kidney Disease in Pregnancy. In: Brenner and Rector’s the Kidney, edited by Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu AS, 10th Ed., Philadelphia, Elsevier, 2016
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee : Executive summary: Heart disease and stroke statistics--2016 update: A report from the American heart association. Circulation 133: 447–454, 2016 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

