First Selective 12-LOX Inhibitor, ML355, Impairs Thrombus Formation and Vessel Occlusion In Vivo With Minimal Effects on Hemostasis
- PMID: 28775075
- PMCID: PMC5620123
- DOI: 10.1161/ATVBAHA.117.309868
First Selective 12-LOX Inhibitor, ML355, Impairs Thrombus Formation and Vessel Occlusion In Vivo With Minimal Effects on Hemostasis
Abstract
Objective: Adequate platelet reactivity is required for maintaining hemostasis. However, excessive platelet reactivity can also lead to the formation of occlusive thrombi. Platelet 12(S)-lipoxygenase (12-LOX), an oxygenase highly expressed in the platelet, has been demonstrated to regulate platelet function and thrombosis ex vivo, supporting a key role for 12-LOX in the regulation of in vivo thrombosis. However, the ability to pharmacologically target 12-LOX in vivo has not been established to date. Here, we studied the effect of the first highly selective 12-LOX inhibitor, ML355, on in vivo thrombosis and hemostasis.
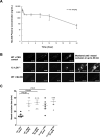
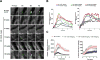
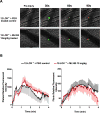
Approach and results: ML355 dose-dependently inhibited human platelet aggregation and 12-LOX oxylipin production, as confirmed by mass spectrometry. Interestingly, the antiplatelet effects of ML355 were reversed after exposure to high concentrations of thrombin in vitro. Ex vivo flow chamber assays confirmed that human platelet adhesion and thrombus formation at arterial shear over collagen were attenuated in whole blood treated with ML355 comparable to aspirin. Oral administration of ML355 in mice showed reasonable plasma drug levels by pharmacokinetic assessment. ML355 treatment impaired thrombus growth and vessel occlusion in FeCl3-induced mesenteric and laser-induced cremaster arteriole thrombosis models in mice. Importantly, hemostatic plug formation and bleeding after treatment with ML355 was minimal in mice in response to laser ablation on the saphenous vein or in a cremaster microvasculature laser-induced rupture model.
Conclusions: Our data strongly support 12-LOX as a key determinant of platelet reactivity in vivo, and inhibition of platelet 12-LOX with ML355 may represent a new class of antiplatelet therapy.
Keywords: collagen; hemostasis; lipoxygenase; platelet reactivity; thrombosis.
© 2017 American Heart Association, Inc.
Figures




References
-
- Ruggeri ZM. Platelets in atherothrombosis. Nature medicine. 2002;8:1227–1234. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:399–410. - PubMed
-
- Steg PG, Dorman SH, Amarenco P. Atherothrombosis and the role of antiplatelet therapy. J Thromb Haemost. 2011;9(Suppl 1):325–332. - PubMed
-
- Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

