Chronic Rejection of Cardiac Allografts Is Associated With Increased Lymphatic Flow and Cellular Trafficking
- PMID: 28775077
- PMCID: PMC5737875
- DOI: 10.1161/CIRCULATIONAHA.117.028533
Chronic Rejection of Cardiac Allografts Is Associated With Increased Lymphatic Flow and Cellular Trafficking
Abstract
Background: Cardiac transplantation is an excellent treatment for end-stage heart disease. However, rejection of the donor graft, in particular, by chronic rejection leading to cardiac allograft vasculopathy, remains a major cause of graft loss. The lymphatic system plays a crucial role in the alloimmune response, facilitating trafficking of antigen-presenting cells to draining lymph nodes. The encounter of antigen-presenting cells with T lymphocytes in secondary lymphoid organs is essential for the initiation of alloimmunity. Donor lymphatic vessels are not anastomosed to that of the recipient during transplantation. The pathophysiology of lymphatic disruption is unknown, and whether this disruption enhances or hinders the alloimmune responses is unclear. Although histological analysis of lymphatic vessels in donor grafts can yield information on the structure of the lymphatics, the function following cardiac transplantation is poorly understood.
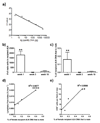
Methods: Using single-photon emission computed tomography/computed tomography lymphoscintigraphy, we quantified the lymphatic flow index following heterotrophic cardiac transplantation in a murine model of chronic rejection.
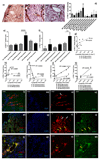
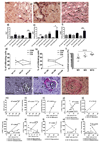
Results: Ten weeks following transplantation of a minor antigen (HY) sex-mismatched heart graft, the lymphatic flow index was significantly increased in comparison with sex-matched controls. Furthermore, the enhanced lymphatic flow index correlated with an increase in donor cells in the mediastinal draining lymph nodes; increased lymphatic vessel area; and graft infiltration of CD4+, CD8+ T cells, and CD68+ macrophages.
Conclusions: Chronic rejection results in increased lymphatic flow from the donor graft to draining lymph nodes, which may be a factor in promoting cellular trafficking, alloimmunity, and cardiac allograft vasculopathy.
Keywords: immune system; inflammation; rejection; transplantation.
© 2017 American Heart Association, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose
Figures

Comment in
-
Lymphangiogenesis in Chronic Rejection and Coronary Allograft Vasculopathy: An Emerging Diagnostic and Therapeutic Target?Circulation. 2018 Jan 30;137(5):504-507. doi: 10.1161/CIRCULATIONAHA.117.031716. Circulation. 2018. PMID: 29378756 Free PMC article. No abstract available.
References
-
- Taylor R, Lannon J, Wong E, Collett D. Annual Report on Cardiothoracic Transplantation. Statistics & Clinical Studies, NHS Blood & Transplant. 2015:1–131. http://www.odt.nhs.uk/pdf/organ_specific_report_cardiothoracic_2015.pdf.
-
- Sayegh MH, Carpenter CB. Transplantation 50 years later--progress, challenges, and promises. N Engl J Med. 2004;23:2761–6. - PubMed
-
- Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report 2014; Focus Theme: Retransplantation. The Journal of Heart and Lung Transplantation. 2014;33:996–1008. - PubMed
-
- Nykanen AI, Sandelin H, Krebs R, Keranen MA, Tuuminen R, Karpanen T, Wu Y, Pytowski B, Koskinen PK, Yla-Herttuala S, Alitalo K, et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–22. - PubMed
-
- Hasegawa T, Visovatti SH, Hyman MC, Hayasaki T, Pinsky DJ. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nat Protoc. 2007;2:471–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

