Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae
- PMID: 28775098
- PMCID: PMC5792181
- DOI: 10.1152/ajplung.00244.2017
Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae
Abstract
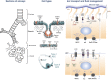
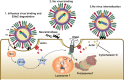
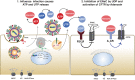
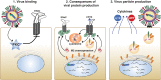
The cystic fibrosis transmembrane conductance regulator (CFTR) and the amiloride-sensitive epithelial sodium channels (ENaC) are located in the apical membranes of airway and alveolar epithelial cells. These transporters play an important role in the regulation of lung fluid balance across airway and alveolar epithelia by being the conduits for chloride (Cl-) and bicarbonate ([Formula: see text]) secretion and sodium (Na+) ion absorption, respectively. The functional role of these channels in the respiratory tract is to maintain the optimum volume and ionic composition of the bronchial periciliary fluid (PCL) and alveolar lining fluid (ALF) layers. The PCL is required for proper mucociliary clearance of pathogens and debris, and the ALF is necessary for surfactant homeostasis and optimum gas exchange. Dysregulation of ion transport may lead to mucus accumulation, bacterial infections, inflammation, pulmonary edema, and compromised respiratory function. Influenza (or flu) in mammals is caused by influenza A and B viruses. Symptoms include dry cough, sore throat, and is often followed by secondary bacterial infections, accumulation of fluid in the alveolar spaces and acute lung injury. The underlying mechanisms of flu symptoms are not fully understood. This review summarizes our present knowledge of how influenza virus infections alter airway and alveolar epithelial cell CFTR and ENaC function in vivo and in vitro and the role of these changes in influenza pathogenesis.
Keywords: M2 protein; Na+/K+-ATPase; calcium-activated Cl− channels; cystic fibrosis transmembrane conductance regulator; epithelial sodium channels.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Aeffner F, Abdulrahman B, Hickman-Davis JM, Janssen PM, Amer A, Bedwell DM, Sorscher EJ, Davis IC. Heterozygosity for the F508del mutation in the cystic fibrosis transmembrane conductance regulator anion channel attenuates influenza severity. J Infect Dis 208: 780–789, 2013. doi: 10.1093/infdis/jit251. - DOI - PMC - PubMed
-
- Åstrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol 308: L22–L32, 2015. doi: 10.1152/ajplung.00163.2014. - DOI - PMC - PubMed
-
- Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol 277: L694–L699, 1999. - PubMed
-
- Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl− secretion in airway epithelia. J Biol Chem 277: 43041–43049, 2002. doi: 10.1074/jbc.M203154200. - DOI - PubMed
-
- Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, Lin V, Shastry S, Mazur M, Sloane PA, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 310: L928–L939, 2016. doi: 10.1152/ajplung.00395.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

