OsbZIP48, a HY5 Transcription Factor Ortholog, Exerts Pleiotropic Effects in Light-Regulated Development
- PMID: 28775143
- PMCID: PMC5813549
- DOI: 10.1104/pp.17.00478
OsbZIP48, a HY5 Transcription Factor Ortholog, Exerts Pleiotropic Effects in Light-Regulated Development
Abstract
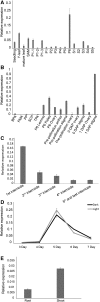
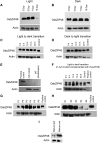
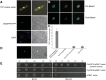
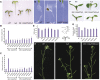
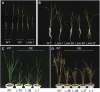
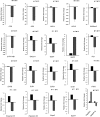
Plants have evolved an intricate network of sensory photoreceptors and signaling components to regulate their development. Among the light signaling components identified to date, HY5, a basic leucine zipper (bZIP) transcription factor, has been investigated extensively. However, most of the work on HY5 has been carried out in Arabidopsis (Arabidopsis thaliana), a dicot. In this study, based on homology search and phylogenetic analysis, we identified three homologs of AtHY5 in monocots; however, AtHYH (HY5 homolog) homologs are absent in the monocots analyzed. Out of the three homologs identified in rice (Oryza sativa), we have functionally characterized OsbZIP48OsbZIP48 was able to complement the Athy5 mutant. OsbZIP48 protein levels are developmentally regulated in rice. Moreover, the OsbZIP48 protein does not degrade in dark-grown rice and Athy5 seedlings complemented with OsbZIP48, which is in striking contrast to AtHY5. In comparison with AtHY5, which does not cause any change in hypocotyl length when overexpressed in Arabidopsis, the overexpression of full-length OsbZIP48 in rice transgenics reduced the plant height considerably. Microarray analysis revealed that OsKO2, which encodes ent-kaurene oxidase 2 of the gibberellin biosynthesis pathway, is down-regulated in OsbZIP48OE and up-regulated in OsbZIP48KD transgenics as compared with the wild type. Electrophoretic mobility shift assay showed that OsbZIP48 binds directly to the OsKO2 promoter. The RNA interference lines and the T-DNA insertional mutant of OsbZIP48 showed seedling-lethal phenotypes despite the fact that roots were more proliferative during early stages of development in the T-DNA insertional mutant. These data provide credible evidence that OsbZIP48 performs more diverse functions in a monocot system like rice in comparison with its Arabidopsis ortholog, HY5.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Alabadí D, Gallego-Bartolomé J, Orlando L, García-Cárcel L, Rubio V, Martínez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, et al. (2008) Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J 53: 324–335 - PubMed
-
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 - PubMed
-
- Bandyopadhyay A, Blakeslee JJ, Lee OR, Mravec J, Sauer M, Titapiwatanakun B, Makam SN, Bouchard R, Geisler M, Martinoia E, et al. (2007) Interactions of PIN and PGP auxin transport mechanisms. Biochem Soc Trans 35: 137–141 - PubMed
-
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA (1996) Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273: 948–950 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

