Loss of Igf2 Gene Imprinting in Murine Prostate Promotes Widespread Neoplastic Growth
- PMID: 28775169
- PMCID: PMC9741865
- DOI: 10.1158/0008-5472.CAN-16-3089
Loss of Igf2 Gene Imprinting in Murine Prostate Promotes Widespread Neoplastic Growth
Abstract
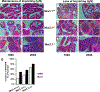
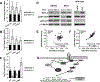
Loss of imprinting (LOI) is an epigenetic event that relaxes an allele-specific restriction on gene expression. One gene that experiences LOI is the paracrine insulin-like growth factor IGF2, which occurs commonly in human prostate tissues during aging and tumorigenesis. However, the relationship between IGF2 LOI and prostate tumorigenesis has not been established functionally. In this study, we created a mouse model with CTCF-binding site mutations at the Igf2-H19 imprint control region that abolishes CTCF insulator activity, resulting in biallelic Igf2 expression that mimics increased levels seen with aging-induced LOI. We found that Igf2 LOI increased the prevalence and severity of prostatic intraepithelial neoplasia (PIN), a premalignant lesion. Engineering Nkx3.1 deficiency into our model increased the frequency of PIN lesions in an additive fashion. Prostates harboring LOI displayed increased MAPK signaling and epithelial proliferation. In human prostate tissue arrays, we documented a positive correlation in benign tissues of IGF2 levels with phospho-ERK and phospho-AKT levels. Overall, our results establish that Igf2 LOI is sufficient on its own to increase rates of neoplastic development in the prostate by upregulating critical cancer-associated signaling pathways. Cancer Res; 77(19); 5236-47. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures



Comment in
-
Prostate cancer: IGF2 imprinting loss promotes cancer.Nat Rev Urol. 2017 Oct;14(10):583. doi: 10.1038/nrurol.2017.148. Epub 2017 Aug 31. Nat Rev Urol. 2017. PMID: 28858333 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29 - PubMed
-
- Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J Natl Cancer Inst 1980;65:311–6 - PubMed
-
- Jarrard DF, Bussemakers MJ, Bova GS, Isaacs WB. Regional loss of imprinting of the insulin-like growth factor II gene occurs in human prostate tissues. Clin Cancer Res 1995;1:1471–8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

