Solution nuclear magnetic resonance spectroscopy on a nanostructured diamond chip
- PMID: 28775280
- PMCID: PMC5543112
- DOI: 10.1038/s41467-017-00266-4
Solution nuclear magnetic resonance spectroscopy on a nanostructured diamond chip
Abstract
Sensors using nitrogen-vacancy centers in diamond are a promising tool for small-volume nuclear magnetic resonance (NMR) spectroscopy, but the limited sensitivity remains a challenge. Here we show nearly two orders of magnitude improvement in concentration sensitivity over previous nitrogen-vacancy and picoliter NMR studies. We demonstrate NMR spectroscopy of picoliter-volume solutions using a nanostructured diamond chip with dense, high-aspect-ratio nanogratings, enhancing the surface area by 15 times. The nanograting sidewalls are doped with nitrogen-vacancies located a few nanometers from the diamond surface to detect the NMR spectrum of roughly 1 pl of fluid lying within adjacent nanograting grooves. We perform 1H and 19F nuclear magnetic resonance spectroscopy at room temperature in magnetic fields below 50 mT. Using a solution of CsF in glycerol, we determine that 4 ± 2 × 1012 19F spins in a 1 pl volume can be detected with a signal-to-noise ratio of 3 in 1 s of integration.Nitrogen vacancy (NV) centres in diamond can be used for NMR spectroscopy, but increased sensitivity is needed to avoid long measurement times. Kehayias et al. present a nanostructured diamond grating with a high density of NV centres, enabling NMR spectroscopy of picoliter-volume solutions.
Conflict of interest statement
A.J., D.B., and L.B. are co-founders of startup ODMR Technologies and have financial interests in the company. The remaining authors declare no competing financial interests.
Figures

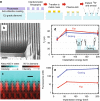
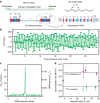

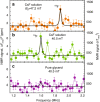
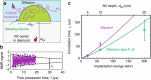
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

