Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews
- PMID: 28775845
- PMCID: PMC5539638
- DOI: 10.1186/s13601-017-0160-0
Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews
Abstract
Background: There is clinical uncertainty about the effectiveness and safety of allergen immunotherapy (AIT) for the treatment of allergic asthma.
Objectives: To undertake a systematic overview of the effectiveness, cost-effectiveness and safety of AIT for the treatment of allergic asthma.
Methods: We searched nine electronic databases from inception to October 31, 2015. Systematic reviews were independently screened by two reviewers against pre-defined eligibility criteria and critically appraised using the Critical Appraisal Skills Programme quality assessment tool for systematic reviews. Data were descriptively and thematically synthesized.
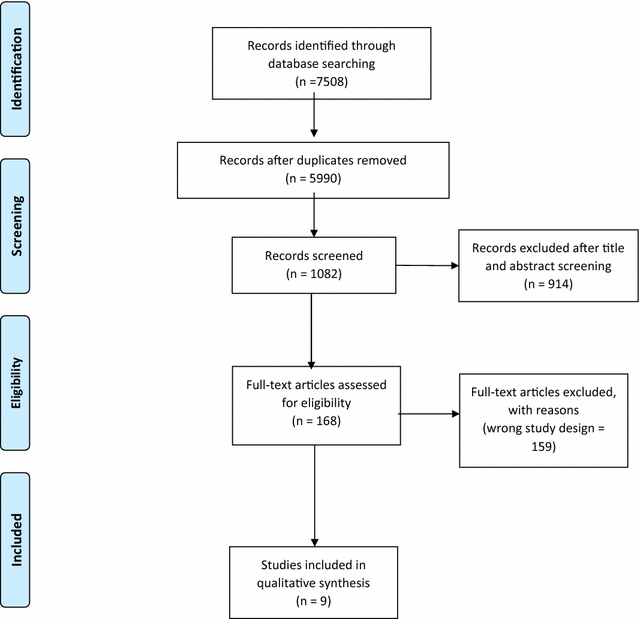
Results: We identified nine eligible systematic reviews; these focused on delivery of AIT through the following routes: subcutaneous (SCIT; n = 3); sublingual (SLIT; n = 4); and both SCIT and SLIT (n = 2). This evidence found that AIT delivered by SCIT and SLIT can improve medication and symptom scores and measures of bronchial hyper-reactivity. The impact on measures of lung function or asthma control was however less clear. We found no systematic review level evidence on the cost-effectiveness of SCIT or SLIT. SLIT had a favorable safety profile when compared to SCIT, particularly in relation to the risk of systemic reactions.
Conclusions: AIT has the potential to achieve reductions in symptom and medication scores, but there is no clear or consistent evidence that measures of lung function can be improved. Bearing in mind the limitations of synthesizing evidence from systematic reviews and the fact that these reviews include mainly dated studies, a systematic review of current primary studies is now needed to update this evidence base, estimate the effectiveness of AIT on asthma outcomes and to investigate the relative effectiveness, cost-effectiveness and safety of SCIT and SLIT.
References
-
- Report TGA. Global burden of disease due to Asthma. 2014; http://www.globalasthmareport.org/burden/burden.php.
-
- Organization WH. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach; 2007.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources