Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes
- PMID: 28776010
- PMCID: PMC5532721
- DOI: 10.1021/acscentsci.7b00212
Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes
Abstract
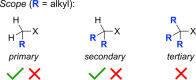
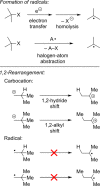
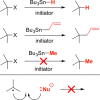
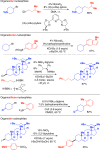
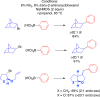
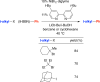
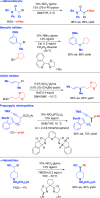
Classical methods for achieving nucleophilic substitutions of alkyl electrophiles (SN1 and SN2) have limited scope and are not generally amenable to enantioselective variants that employ readily available racemic electrophiles. Radical-based pathways catalyzed by chiral transition-metal complexes provide an attractive approach to addressing these limitations.
Conflict of interest statement
The author declares no competing financial interest.
Figures



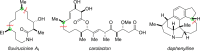
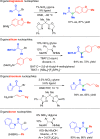


References
-
- Hartshorn S. R.Aliphatic Nucleophilic Substitution; Cambridge University Press: London, 1973.
-
- Anslyn E. V.; Dougherty D. A.. Substitutions at Aliphatic Centers and Thermal Isomerizations/Rearrangements. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, 2006; Chapter 11.
-
- For examples of interesting exceptions (albeit with limited scope with respect to the nucleophile and the electrophile), see:Reisman S. E.; Doyle A. G.; Jacobsen E. N. Enantioselective Thiourea-Catalyzed Additions to Oxocarbenium Ions. J. Am. Chem. Soc. 2008, 130, 7198–7199. 10.1021/ja801514m. - DOI - PMC - PubMed
-
- Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu C., Studer A., Eds.; John Wiley & Sons, Chichester, U.K., 2012; Vols. 1 and 2.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

