1,6-Cyclophellitol Cyclosulfates: A New Class of Irreversible Glycosidase Inhibitor
- PMID: 28776021
- PMCID: PMC5532717
- DOI: 10.1021/acscentsci.7b00214
1,6-Cyclophellitol Cyclosulfates: A New Class of Irreversible Glycosidase Inhibitor
Abstract
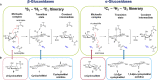
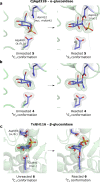
The essential biological roles played by glycosidases, coupled to the diverse therapeutic benefits of pharmacologically targeting these enzymes, provide considerable motivation for the development of new inhibitor classes. Cyclophellitol epoxides and aziridines are recently established covalent glycosidase inactivators. Inspired by the application of cyclic sulfates as electrophilic equivalents of epoxides in organic synthesis, we sought to test whether cyclophellitol cyclosulfates would similarly act as irreversible glycosidase inhibitors. Here we present the synthesis, conformational analysis, and application of novel 1,6-cyclophellitol cyclosulfates. We show that 1,6-epi-cyclophellitol cyclosulfate (α-cyclosulfate) is a rapidly reacting α-glucosidase inhibitor whose 4C1 chair conformation matches that adopted by α-glucosidase Michaelis complexes. The 1,6-cyclophellitol cyclosulfate (β-cyclosulfate) reacts more slowly, likely reflecting its conformational restrictions. Selective glycosidase inhibitors are invaluable as mechanistic probes and therapeutic agents, and we propose cyclophellitol cyclosulfates as a valuable new class of carbohydrate mimetics for application in these directions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Epi-Cyclophellitol Cyclosulfate, a Mechanism-Based Endoplasmic Reticulum α-Glucosidase II Inhibitor, Blocks Replication of SARS-CoV-2 and Other Coronaviruses.ACS Cent Sci. 2024 Jul 25;10(8):1594-1608. doi: 10.1021/acscentsci.4c00506. eCollection 2024 Aug 28. ACS Cent Sci. 2024. PMID: 39220688 Free PMC article.
-
Conformational and Electronic Variations in 1,2- and 1,5a-Cyclophellitols and their Impact on Retaining α-Glucosidase Inhibition.Chemistry. 2024 Jun 3;30(31):e202400723. doi: 10.1002/chem.202400723. Epub 2024 Apr 24. Chemistry. 2024. PMID: 38623783
-
New Irreversible α-l-Iduronidase Inhibitors and Activity-Based Probes.Chemistry. 2018 Dec 17;24(71):19081-19088. doi: 10.1002/chem.201804662. Epub 2018 Nov 26. Chemistry. 2018. PMID: 30307091 Free PMC article.
-
Mechanism-based inhibitors of glycosidases: design and applications.Adv Carbohydr Chem Biochem. 2014;71:297-338. doi: 10.1016/B978-0-12-800128-8.00004-2. Adv Carbohydr Chem Biochem. 2014. PMID: 25480507 Review.
-
Back to (non-)Basics: An Update on Neutral and Charge-Balanced Glycosidase Inhibitors.Mini Rev Med Chem. 2018;18(10):812-827. doi: 10.2174/1389557517666171002161325. Mini Rev Med Chem. 2018. PMID: 28969552 Review.
Cited by
-
Distinguishing the differences in β-glycosylceramidase folds, dynamics, and actions informs therapeutic uses.J Lipid Res. 2018 Dec;59(12):2262-2276. doi: 10.1194/jlr.R086629. Epub 2018 Oct 2. J Lipid Res. 2018. PMID: 30279220 Free PMC article.
-
Dynamic and Functional Profiling of Xylan-Degrading Enzymes in Aspergillus Secretomes Using Activity-Based Probes.ACS Cent Sci. 2019 Jun 26;5(6):1067-1078. doi: 10.1021/acscentsci.9b00221. Epub 2019 May 24. ACS Cent Sci. 2019. PMID: 31263766 Free PMC article.
-
Glycosylated cyclophellitol-derived activity-based probes and inhibitors for cellulases.RSC Chem Biol. 2020 Jul 28;1(3):148-155. doi: 10.1039/d0cb00045k. eCollection 2020 Aug 1. RSC Chem Biol. 2020. PMID: 34458755 Free PMC article.
-
Activity-based protein profiling reveals dynamic substrate-specific cellulase secretion by saprotrophic basidiomycetes.Biotechnol Biofuels Bioprod. 2022 Jan 17;15(1):6. doi: 10.1186/s13068-022-02107-z. Biotechnol Biofuels Bioprod. 2022. PMID: 35418096 Free PMC article.
-
Xylose-Configured Cyclophellitols as Selective Inhibitors for Glucocerebrosidase.Chembiochem. 2021 Nov 3;22(21):3090-3098. doi: 10.1002/cbic.202100396. Epub 2021 Sep 13. Chembiochem. 2021. PMID: 34459538 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

