Inhibition of dengue virus replication by novel inhibitors of RNA-dependent RNA polymerase and protease activities
- PMID: 28776445
- PMCID: PMC6010079
- DOI: 10.1080/14756366.2017.1355791
Inhibition of dengue virus replication by novel inhibitors of RNA-dependent RNA polymerase and protease activities
Abstract
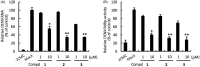
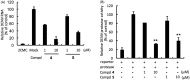
Dengue virus (DENV) is the leading mosquito-transmitted viral infection in the world. With more than 390 million new infections annually, and up to 1 million clinical cases with severe disease manifestations, there continues to be a need to develop new antiviral agents against dengue infection. In addition, there is no approved anti-DENV agents for treating DENV-infected patients. In the present study, we identified new compounds with anti-DENV replication activity by targeting viral replication enzymes - NS5, RNA-dependent RNA polymerase (RdRp) and NS3 protease, using cell-based reporter assay. Subsequently, we performed an enzyme-based assay to clarify the action of these compounds against DENV RdRp or NS3 protease activity. Moreover, these compounds exhibited anti-DENV activity in vivo in the ICR-suckling DENV-infected mouse model. Combination drug treatment exhibited a synergistic inhibition of DENV replication. These results describe novel prototypical small anti-DENV molecules for further development through compound modification and provide potential antivirals for treating DENV infection and DENV-related diseases.
Keywords: DENV inhibitors; ICR-suckling mouse; NS3 protease; RdRp; synergy.
Figures



References
-
- Rothman AL.Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 2011;11:532–43. - PubMed
-
- Halstead SH.Dengue virus-mosquito interactions. Annu Rev Entomol 2008;53:273–91. - PubMed
-
- Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ.. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep 2010;12:157–64. - PubMed
-
- Roche C, Cassar O, Laille M, Murgue B.. Dengue-3 virus genomic differences that correlate with in vitro phenotype on a human cell line but not with disease severity. Microbes Infect 2007;9:63–9. - PubMed
-
- Shurtleff AC, Beasley DW, Chen JJ, et al. Genetic variation in the 3' non-coding region of dengue viruses. Virology 2001;281:75–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
