Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies
- PMID: 28776876
- PMCID: PMC5623751
- DOI: 10.1111/cas.13343
Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies
Abstract
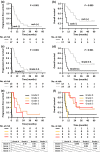
The present study sought to elucidate the prognosis of adult T-cell leukemia-lymphoma (ATL) patients receiving mogamulizumab, a defucosylated anti-CCR4 monoclonal antibody. Progression-free survival (PFS) and overall survival (OS) of ATL patients enrolled in two studies are herein updated, namely NCT00355472 (phase I study of mogamulizumab in relapsed patients with ATL and peripheral T-cell lymphoma) and NCT00920790 (phase II study for relapsed ATL). Of 13 patients with relapsed aggressive ATL in the phase I study, four (31%) survived >3 years. For 26 relapsed patients with aggressive ATL in the phase II study, median PFS was 5.2 months and 1-year PFS was 26%, whereas median OS was 14.4 months, and 3-year OS was 23%. For patients without a rash or who developed a grade 1 rash only, median PFS was 0.8 months, and 1-year PFS was zero, with a median OS of 6.0 months, and 3-year OS of 8%. In contrast, for patients who developed a rash ≥grade 2, median PFS was 11.7 months, and 1-year PFS was 50%, with a median OS of 25.6 months, and 3-year OS of 36%. Thus, we conclude that mogamulizumab monotherapy may improve PFS and OS in some patients with relapsed aggressive ATL, especially those who develop a skin rash as a moderate immune-related adverse event. Therefore, further investigation is warranted to validate the present observations and to clarify the mechanisms involved in the activity of mogamulizumab.
Keywords: Adult T-cell leukemia-lymphoma; CC chemokine receptor 4; mogamulizumab; rash; regulatory T cell.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Vose J, Armitage J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–30. - PubMed
-
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma: a report from the Lymphoma Study Group (1984–87). Br J Haematol 1991; 79: 428–37. - PubMed
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1997; 50: 481–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

