Enantioselective Crossed Photocycloadditions of Styrenic Olefins by Lewis Acid Catalyzed Triplet Sensitization
- PMID: 28776908
- PMCID: PMC5661956
- DOI: 10.1002/anie.201706975
Enantioselective Crossed Photocycloadditions of Styrenic Olefins by Lewis Acid Catalyzed Triplet Sensitization
Abstract
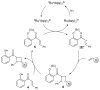
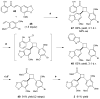
The synthesis of unsymmetrical cyclobutanes by controlled heterodimerization of olefins remains a substantial challenge, particularly in an enantiocontrolled fashion. Shown herein is that chiral Lewis acid catalyzed triplet sensitization enables the synthesis of highly enantioenriched diarylcyclobutanes by photocycloaddition of structurally varied 2'-hydroxychalcones with a range of styrene coupling partners. The utility of this reaction is demonstrated through the direct synthesis of a representative norlignan cyclobutane natural product.
Keywords: asymmetric catalysis; cycloaddition; cyclobutanes; photocatalysis; ruthenium.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
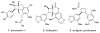

References
-
-
For recent reviews, see: Xu Y, Conner ML, Brown MK. Angew Chem Int Ed. 2015;54:11918–11928.Poplata S, Tröster A, Zou Y, Bach T. Chem Rev. 2016;116:9748–9815.
-
-
-
For recent reviews, see: Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chemie Int Ed. 2002;41:1668–1698.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977–1050.Grover HK, Emmett MR, Kerr MA. Org Biomol Chem. 2015;13:655–671.Klier L, Tur F, Poulsen PH, Jørgensen KA. Chem Soc Rev. 2017;46:1080–1102.
-
-
- Hayashi Y, Narasaka K. Chem Lett. 1989:793–796.
- Canales E, Corey EJ. J Am Chem Soc. 2007;129:12686–12687. - PubMed
- Suárez-Pantiga S, Hernández-Díaz C, Rubio E, González JM. Angew Chem, Int Ed. 2012;51:11552–11555. - PubMed
- Conner ML, Xu Y, Brown MK. J Am Chem Soc. 2015;137:3482–3845. - PubMed
- Hu JL, Feng LW, Wang L, Xie Z, Tang Y, Li X. J Am Chem Soc. 2016;138:13151–13154. - PubMed
-
- Ishihara K, Nakano K. J Am Chem Soc. 2007;129:8930–8931. - PubMed
- Albrecht L, Dickmeiss G, Acosta FC, Rodríguez-Escrich C, Davis RL, Jørgenson KA. J Am Chem Soc. 2012;134:2543–2546. - PubMed
- Talavera G, Reyes E, Vicario JL, Carillo L. Angew Chem Int Ed. 2012;51:4104–4107. - PubMed
- Duan GJ, Ling JB, Wang WP, Luo YC, Xu PF. Chem Commun. 2013;49:4625–4627. - PubMed
- Qi L, Yang Y, Gui Y, Zhang Y, Chen F, Tian F, Peng L. Org Lett. 2014;16:6436–6439. - PubMed
- Halskov KS, Kniep F, Lauridsen VH, Iversen EH, Donslund BS, Jørgenson KA. J Am Chem Soc. 2015;137:1685–1691. [1] - PubMed
- Nielsen AJ, Jenkins HA, McNulty J. Chem Eur J. 2016;22:9111–9115. - PubMed
-
- Du J, Skubi KL, Schultz DM, Yoon TP. Science. 2014;344:392–396. - PMC - PubMed
- Maturi MM, Bach T. Angew Chem, Int Ed. 2014;53:7661–7664. - PubMed
- Tröster A, Alonso R, Bauer A, Bach T. J Am Chem Soc. 2016;138:7808–7811. - PMC - PubMed
- Coote SC, Pöthig A, Bach T. Chem Eur J. 2015;21:6906–6912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

