The Role of Public-Private Partnerships in Catalyzing the Critical Path
- PMID: 28776943
- PMCID: PMC6402188
- DOI: 10.1111/cts.12488
The Role of Public-Private Partnerships in Catalyzing the Critical Path
Figures
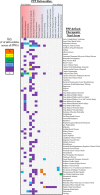
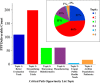
References
-
- US Food and Drug Administration . Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathI... 2017, June 9.
-
- US Food and Drug Administration . Critical Path Opportunities List. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathI... 2017, June 9.
-
- Parekh, A. et al Catalyzing the Critical Path Initiative: FDA's progress in drug development activities. Clin. Pharmacol. Ther. 97, 221–233 (2015). - PubMed
-
- US Food and Drug Administration . CDER Staff Participation in Public Private Partnerships and Consortia. https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalPro... 2017, March 1.
-
- Goodsaid, F.M. & Mendrick, D.L. Translational medicine and the value of biomarker qualification. Sci. Transl. Med. 2, 47ps4 (2010). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

