Electrospun Nanofibers: New Concepts, Materials, and Applications
- PMID: 28777535
- PMCID: PMC6589094
- DOI: 10.1021/acs.accounts.7b00218
Electrospun Nanofibers: New Concepts, Materials, and Applications
Abstract
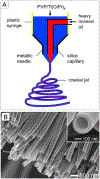
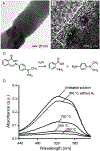
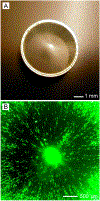
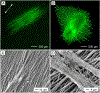
Electrospinning is a simple and versatile technique that relies on the electrostatic repulsion between surface charges to continuously draw nanofibers from a viscoelastic fluid. It has been applied to successfully produce nanofibers, with diameters down to tens of nanometers, from a rich variety of materials, including polymers, ceramics, small molecules, and their combinations. In addition to solid nanofibers with a smooth surface, electrospinning has also been adapted to generate nanofibers with a number of secondary structures, including those characterized by a porous, hollow, or core-sheath structure. The surface and/or interior of such nanofibers can be further functionalized with molecular species or nanoparticles during or after an electrospinning process. In addition, electrospun nanofibers can be assembled into ordered arrays or hierarchical structures by manipulation of their alignment, stacking, and/or folding. All of these attributes make electrospun nanofibers well-suited for a broad spectrum of applications, including those related to air filtration, water purification, heterogeneous catalysis, environmental protection, smart textiles, surface coating, energy harvesting/conversion/storage, encapsulation of bioactive species, drug delivery, tissue engineering, and regenerative medicine. Over the past 15 years, our group has extensively explored the use of electrospun nanofibers for a range of applications. Here we mainly focus on two examples: (i) use of ceramic nanofibers as catalytic supports for noble-metal nanoparticles and (ii) exploration of polymeric nanofibers as scaffolding materials for tissue regeneration. Because of their high porosity, high surface area to volume ratio, well-controlled composition, and good thermal stability, nonwoven membranes made of ceramic nanofibers are terrific supports for catalysts based on noble-metal nanoparticles. We have investigated the use of ceramic nanofibers made of various oxides, including SiO2, TiO2, SnO2, CeO2, and ZrO2, as supports for heterogeneous catalysts based on noble metals such as Au, Pt, Pd, and Rh. On the other hand, the diameter, composition, alignment, porosity, and surface properties of polymeric nanofibers can be engineered in a controllable fashion to mimic the hierarchical architecture of an extracellular matrix and help manipulate cell behaviors for tissue engineering and regenerative medicine. To this end, we can mimic the native structure and morphology of the extracellular matrix in tendon using uniaxially aligned nanofibers; we can use radially aligned nanofibers to direct the migration of cells from the periphery to the center in an effort to speed up wound healing; and we can also use uniaxially aligned nanofibers to guide and expedite the extension of neurites for peripheral nerve repair. Furthermore, we can replicate the anatomic structures at the tendon-to-bone insertion using nanofiber scaffolds with graded mineral coatings. In this Account, we aim to demonstrate the unique capabilities of electrospun nanofibers as porous supports for heterogeneous catalysis and as functional scaffolds for tissue regeneration by concentrating on some of the recent results.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Boys CV On the Production, Properties, and Some Suggested Uses of the Finest Threads. Proc. Phys. Soc. London 1887, 9, 8–19.
-
- Cooley JF Apparatus for Electrically Dispersing Fluids. U.S. Patent 692,631, 1902.
-
- Morton WJ Method of Dispersing Fluid. U.S. Patent 705,691, 1902.
-
- Formhals A; Gastell SR Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 1934.
-
- Formhals A Method and Apparatus for Spinning. U.S. Patent 2,349,950, 1944.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

