Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic
- PMID: 28777791
- PMCID: PMC5747363
- DOI: 10.1038/nm.4381
Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic
Abstract
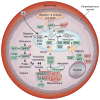
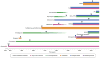
The global adoption of artemisinin-based combination therapies (ACTs) in the early 2000s heralded a new era in effectively treating drug-resistant Plasmodium falciparum malaria. However, several Southeast Asian countries have now reported the emergence of parasites that have decreased susceptibility to artemisinin (ART) derivatives and ACT partner drugs, resulting in increasing rates of treatment failures. Here we review recent advances in understanding how antimalarials act and how resistance develops, and discuss new strategies for effectively combatting resistance, optimizing treatment and advancing the global campaign to eliminate malaria.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- World Health Organization. Fact Sheet: World Malaria Report 2016. WHO; 2016. available at http://www.who.int/malaria/media/world-malaria-report-2016/en/
-
- White NJ, et al. Malaria. Lancet. 2014;383:723–735. - PubMed
-
- Sigala PA, Goldberg DE. The peculiarities and paradoxes of Plasmodium heme metabolism. Annu Rev Microbiol. 2014;68:259–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

