Effect of Methotrexate on an In Vitro Patient-Derived Model of Proliferative Vitreoretinopathy
- PMID: 28777835
- PMCID: PMC5544356
- DOI: 10.1167/iovs.16-20912
Effect of Methotrexate on an In Vitro Patient-Derived Model of Proliferative Vitreoretinopathy
Abstract
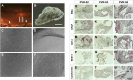
Purpose: The purpose of this study was to develop a method for isolating, culturing, and characterizing cells from patient-derived membranes in proliferative vitreoretinopathy (PVR) to be used for drug testing.
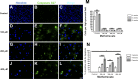
Methods: PVR membranes were obtained from six patients with grade C PVR. Membrane fragments were analyzed by gross evaluation, fixed for immunohistologic studies to establish cell identity, or digested with collagenase II to obtain single cell suspensions for culture. PVR-derived primary cultures were used to examine the effects of methotrexate (MTX) on proliferation, migration, and cell death.
Results: Gross analysis of PVR membranes showed presence of pigmented cells, indicative of retinal pigment epithelial cells. Immunohistochemistry identified cells expressing α-smooth muscle actin, glial fibrillary acidic protein, Bestrophin-1, and F4/80, suggesting the presence of multiple cell types in PVR. Robust PVR primary cultures (C-PVR) were successfully obtained from human membranes, and these cells retained the expression of cell identity markers in culture. C-PVR cultures formed membranes and band-like structures in culture reminiscent of the human condition. MTX significantly reduced the proliferation and band formation of C-PVR, whereas it had no significant effect on cell migration. MTX also induced regulated cell death within C-PVR as assessed by increased expression of caspase-3/7.
Conclusions: PVR cells obtained from human membranes can be successfully isolated, cultured, and profiled in vitro. Using these primary cultures, we identified MTX as capable of significantly reducing growth and inducing cell death of PVR cells in vitro.
Figures




References
-
- Weichel ED, Colyer MH. . Combat ocular trauma and systemic injury. Curr Opin Ophthalmol. 2008; 19: 519– 525. - PubMed
-
- Colyer MH, Chun DW, Bower KS, Dick JS, Weichel ED. . Perforating globe injuries during operation Iraqi Freedom. Ophthalmology. 2008; 115: 2087– 2093. - PubMed
-
- Eliott D, Stryjewski TP, Andreoli MT, Andreoli CM. . Smoking is a risk factor for proliferative vitreoretinopathy after traumatic retinal detachment. Retina. 2017; 37: 1229– 1235. - PubMed
-
- Martini B. . Proliferative vitreo-retinal disorders: experimental models in vivo and in vitro. Acta Ophthalmol Suppl. 1992; 201: 1– 63. - PubMed
-
- The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983; 90: 121– 125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

