Engineered Epidermal Progenitor Cells Can Correct Diet-Induced Obesity and Diabetes
- PMID: 28777946
- PMCID: PMC5555372
- DOI: 10.1016/j.stem.2017.06.016
Engineered Epidermal Progenitor Cells Can Correct Diet-Induced Obesity and Diabetes
Abstract
Somatic gene therapy is a promising approach for treating otherwise terminal or debilitating diseases. The human skin is a promising conduit for genetic engineering, as it is the largest and most accessible organ, epidermal autografts and tissue-engineered skin equivalents have been successfully deployed in clinical applications, and skin epidermal stem/progenitor cells for generating such grafts are easy to obtain and expand in vitro. Here, we develop skin grafts from mouse and human epidermal progenitors that were engineered by CRISPR-mediated genome editing to controllably release GLP-1 (glucagon-like peptide 1), a critical incretin that regulates blood glucose homeostasis. GLP-1 induction from engineered mouse cells grafted onto immunocompetent hosts increased insulin secretion and reversed high-fat-diet-induced weight gain and insulin resistance. Taken together, these results highlight the clinical potential of developing long-lasting, safe, and versatile gene therapy approaches based on engineering epidermal progenitor cells.
Keywords: CRISPR; cutaneous gene therapy; diabetes; epidermal progenitor cells; obesity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
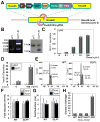


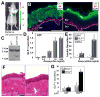
Comment in
-
Engineering Skin with Skinny Genes.Cell Stem Cell. 2017 Aug 3;21(2):153-155. doi: 10.1016/j.stem.2017.07.013. Cell Stem Cell. 2017. PMID: 28777938
References
-
- Carsin H, Ainaud P, Le Bever H, Rives J, Lakhel A, Stephanazzi J, Lambert F, Perrot J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns: journal of the International Society for Burn Injuries. 2000;26:379–387. - PubMed
-
- Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KN, Faucher LD, Caruso DM, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Annals of surgery. 2011;253:672–683. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

