Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents
- PMID: 28778181
- PMCID: PMC5544987
- DOI: 10.1186/s13071-017-2261-y
Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents
Abstract
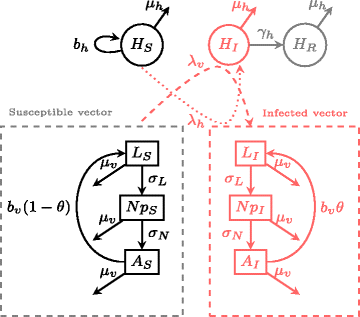
Background: When colonization and gene flow depend on host-mediated dispersal, a key factor affecting vector dispersal potential is the time spent on the host for the blood meal, a characteristic that can vary strongly among life history stages. Using a 2-patch vector-pathogen population model and seabird ticks as biological examples, we explore how vector colonization rates and the spread of infectious agents may be shaped by life stage-dependent dispersal. We contrast hard (Ixodidae) and soft (Argasidae) tick systems, which differ strongly in blood- feeding traits.
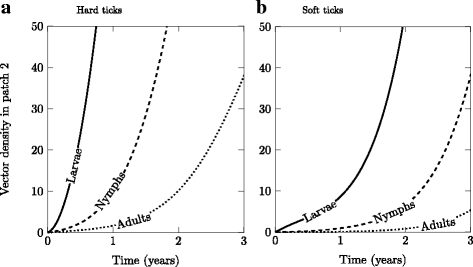
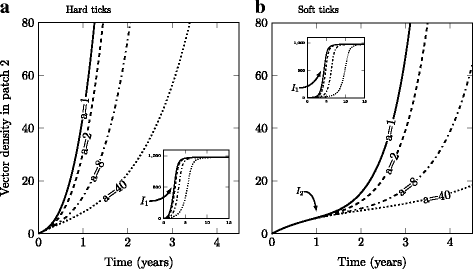
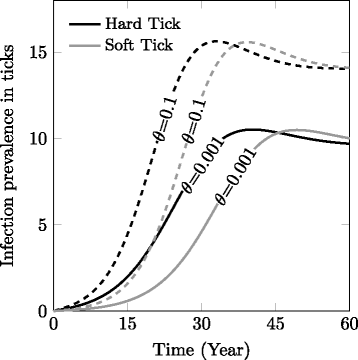
Results: We find that vector life history characteristics (i.e. length of blood meal) and demographic constraints (Allee effects) condition the colonization potential of ticks; hard ticks, which take a single, long blood meal per life stage, should have much higher colonization rates than soft ticks, which take repeated short meals. Moreover, this dispersal potential has direct consequences for the spread of vector-borne infectious agents, in particular when transmission is transovarial.
Conclusions: These results have clear implications for predicting the dynamics of vector and disease spread in the context of large-scale environmental change. The findings highlight the need to include life-stage dispersal in models that aim to predict species and disease distributions, and provide testable predictions related to the population genetic structure of vectors and pathogens along expansion fronts.
Keywords: Allee effect; Borrelia burgdorferi; Climate change; Ixodes uriae; Lyme disease; Ornithodoros maritimus; Parasite spread; Range expansion; Vertical transmission.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Comparison of the lifetime host-to-tick transmission between two strains of the Lyme disease pathogen Borrelia afzelii.Parasit Vectors. 2016 Dec 16;9(1):645. doi: 10.1186/s13071-016-1929-z. Parasit Vectors. 2016. PMID: 27986081 Free PMC article.
-
Which forest bird species are the main hosts of the tick, Ixodes ricinus, the vector of Borrelia burgdorferi sensu lato, during the breeding season?Int J Parasitol. 2012 Jul;42(8):781-8. doi: 10.1016/j.ijpara.2012.05.010. Epub 2012 Jun 23. Int J Parasitol. 2012. PMID: 22732161
-
Host-dependent genetic structure of parasite populations: differential dispersal of seabird tick host races.Evolution. 2003 Feb;57(2):288-96. doi: 10.1111/j.0014-3820.2003.tb00263.x. Evolution. 2003. PMID: 12683525
-
[Ticks (Acarina: Ixodidae) as vectors and reservoirs of pathogen microorganisms in Spain].Enferm Infecc Microbiol Clin. 2005 Feb;23(2):94-102. doi: 10.1157/13071613. Enferm Infecc Microbiol Clin. 2005. PMID: 15743581 Review. Spanish.
-
Tick-host-pathogen interactions in Lyme borreliosis.Trends Parasitol. 2007 Sep;23(9):434-8. doi: 10.1016/j.pt.2007.07.001. Epub 2007 Jul 25. Trends Parasitol. 2007. PMID: 17656156 Review.
Cited by
-
Natal colony influences age-specific movement patterns of the Yellow-legged gull (Larus michahellis).Mov Ecol. 2023 Feb 11;11(1):11. doi: 10.1186/s40462-023-00375-4. Mov Ecol. 2023. PMID: 36774513 Free PMC article.
-
The impact of climate change on ecology of tick associated with tick-borne diseases.PLoS Comput Biol. 2025 Apr 8;21(4):e1012903. doi: 10.1371/journal.pcbi.1012903. eCollection 2025. PLoS Comput Biol. 2025. PMID: 40198742 Free PMC article.
-
Risky business: human-related data is lacking from Lyme disease risk models.Front Public Health. 2023 Nov 3;11:1113024. doi: 10.3389/fpubh.2023.1113024. eCollection 2023. Front Public Health. 2023. PMID: 38026346 Free PMC article. Review.
-
A compartment and metapopulation model of Rocky Mountain spotted fever in southwestern United States and northern Mexico.Infect Dis Model. 2024 Apr 16;9(3):713-727. doi: 10.1016/j.idm.2024.04.008. eCollection 2024 Sep. Infect Dis Model. 2024. PMID: 38659493 Free PMC article.
-
Species-Level Profiling of Ixodes pacificus Bacterial Microbiomes Reveals High Variability Across Short Spatial Scales at Different Taxonomic Resolutions.Genet Test Mol Biomarkers. 2021 Aug;25(8):551-562. doi: 10.1089/gtmb.2021.0088. Genet Test Mol Biomarkers. 2021. PMID: 34406842 Free PMC article.
References
-
- Lemon SM, Sparling PF, Hamburg MA, Relman DA, Choffnes ER, Mack A. Vector-borne diseases: understanding the environmental, human health, and ecological connections, workshop summary. Washington, DC: National Academy of Sciences; 2008. p. 40. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

