Immobilisation of Delta-like 1 ligand for the scalable and controlled manufacture of hematopoietic progenitor cells in a stirred bioreactor
- PMID: 28778182
- PMCID: PMC5544980
- DOI: 10.1186/s12896-017-0383-0
Immobilisation of Delta-like 1 ligand for the scalable and controlled manufacture of hematopoietic progenitor cells in a stirred bioreactor
Abstract
Background: Umbilical cord blood provides a source of hematopoietic stem cells for transplantation with immunological and availability advantages over conventional bone marrow sources. Limited cell numbers and slower engraftment from umbilical cord blood units has led to the clinical development of immobilised Notch ligand Delta-Like 1 to promote ex vivo expansion of a rapidly engrafting cell population. However, current immobilisation methods are not simple to scale in a controlled manner.
Results: Delta-Like 1 was immobilised onto streptavidin coated magnetic particles via a heterobifunctionalised polyethylene glycol linker molecule to provide an easily manipulated format of surface protein presentation. CD34+ enriched cord blood cells were treated with Delta-Like 1 immobilised particles, and immunophenotypic markers measured to monitor population distributions using cluster identification, characterization, and regression software. The amenability of the approach to scalability was evaluated in a micro-scale stirred tank bioreactor. Surface concentration of Delta-Like 1 was well controlled used differing stoichiometric reagent ratios. Protein immobilisation was a cost effective process and particles were efficiently removed from the final cell product. Immobilised Delta-Like 1 is functional and stimulates qualitatively similar CD34hi, CD38lo, CD90lo, CD133hi, CD135hi progenitor expansion in both static culture and scalable stirred culture platforms.
Conclusions: Immobilised Delta-Like 1 in this form has the potential to improve the manufacturing efficiency and control of final ex vivo expanded cell product through compatibility with highly controlled and characterised suspension culture systems.
Keywords: Cell culture; Delta-like 1; Ex vivo expansion; Hematopoietic progenitor cell; Immobilised; Manufacturing; Notch; Scalable.
Conflict of interest statement
Ethics approval and consent to participate
Work was carried out under Anthony Nolan Cell Therapy Centre research tissue bank ethics approval. The ethical approval is explicitly and specifically extended to cover our project work in a material transfer agreement. The approval was extended to non-identifiable tissue from the bank. HTA license number: 22,527.
Consent for publication
Not applicable
Competing interests
Patent pending. Filed 19/05/2016. GB1608847.8.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
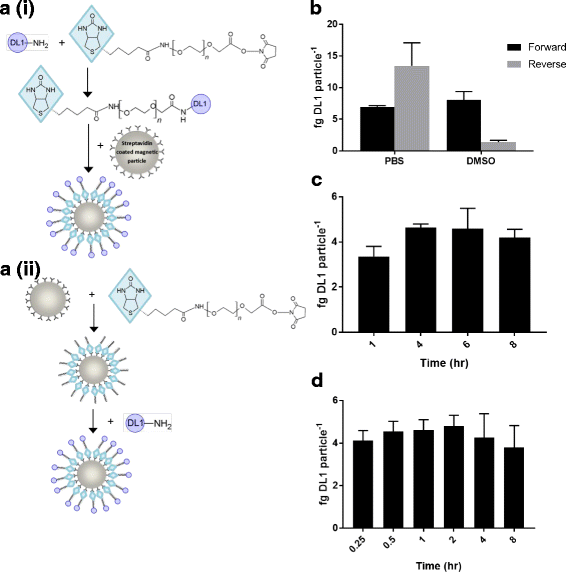
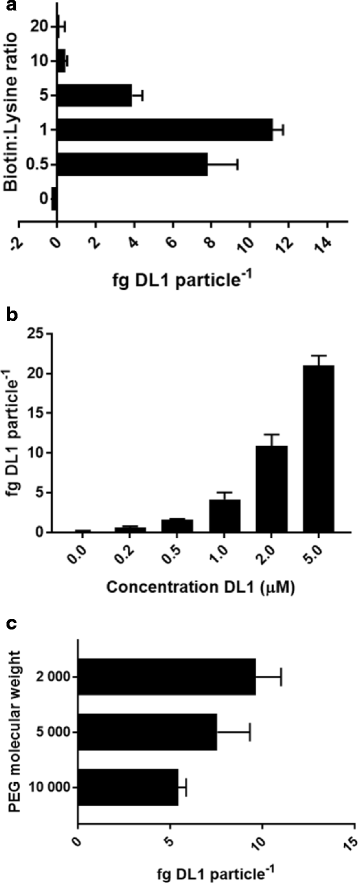



References
-
- Gonzalez-Ryan L, VanSyckle K, Coyne KD, Glover N. Umbilical cord blood banking: procedural and ethical concerns for this new birth option. Pediatr Nurs. 2000;26:105–110. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

