Antimicrobial Efficacy of Triple Antibiotic-eluting Polymer Nanofibers against Multispecies Biofilm
- PMID: 28778504
- PMCID: PMC5657510
- DOI: 10.1016/j.joen.2017.06.009
Antimicrobial Efficacy of Triple Antibiotic-eluting Polymer Nanofibers against Multispecies Biofilm
Abstract
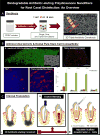
The elimination of microbial flora in cases of immature permanent teeth with necrotic pulp is both key and a challenging goal for the long-term success of regenerative therapy. Recent research has focused on the development of cell-friendly intracanal drug delivery systems. This in vitro study aimed to investigate the antimicrobial action of 3-dimensional (3D) tubular-shaped triple antibiotic-eluting nanofibrous constructs against a multispecies biofilm on human dentin. Polydioxanone polymer solutions, antibiotic-free or incorporated with metronidazole, ciprofloxacin, and minocycline, were electrospun into 3D tubular-shaped constructs. A multispecies biofilm consisting of Actinomyces naeslundii, Streptococcus sanguinis, and Enterococcus faecalis was forced inside the dentinal tubules via centrifugation in a dentin slice in vitro model. The infected specimens were exposed to 2 experimental groups (ie, 3D tubular-shaped triple antibiotic-eluting constructs and triple antibiotic paste [TAP]) and 2 control groups (7-day biofilm untreated and antibiotic-free 3D tubular-shaped constructs). Biofilm elimination was quantitatively analyzed with confocal laser scanning microscopy. Confocal laser scanning microscopic (CLSM) analysis showed a dense population of viable (green) bacteria adhered to dentin and penetrated into the dentinal tubules. Upon 3D tubular-shaped triple antibiotic-eluting nanofibrous construct exposure, nearly complete elimination of viable bacteria on the dentin surface and inside the dentinal tubules was shown in the CLSM images, which was similar (P < .05) to the bacterial death promoted by the TAP group but significantly greater when compared with both the antibiotic-free 3D tubular-shaped constructs and the control (saline). The proposed 3D tubular-shaped antibiotic-eluting construct showed pronounced antimicrobial effects against the multispecies biofilm tested and therefore holds significant clinical potential as a disinfection strategy before regenerative endodontics.
Keywords: Antibiotic; bacteria; disinfection; electrospinning; nanofibers; pulp; regeneration; root canal; scaffold; stem cells.
Copyright © 2017 American Association of Endodontists. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors deny any conflicts of interest related to this study.
Figures


References
-
- Sundqvist G, Figdor D. Life as an endodontic pathogen: Ecological differences between the untreated and root-filled root canals. Endod Topics. 2003;6:3–28.
-
- Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100. - PubMed
-
- Mohammadi Z, Palazzi F, Giardino L, Shalavi S. Microbial biofilms in endodontic infections: an update review. Biomed J. 2013;36:59–70. - PubMed
-
- Jacinto RC, Gomes BP, Desai M, Rajendram D, Shah HN. Bacterial examination of endodontic infections by clonal analysis in concert with denaturing high-performance liquid chromatography. Oral Microbiol Immunol. 2007;22:403–10. - PubMed
-
- Murad CF, Sassone LM, Faveri M, Hirata R, Jr, Figueiredo L, Feres M. Microbial diversity in persistent root canal infections investigated by checkerboard DNA-DNA hybridization. J Endod. 2014;40:899–906. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

