TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections
- PMID: 28778717
- PMCID: PMC7116903
- DOI: 10.1016/j.biochi.2017.07.016
TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections
Abstract
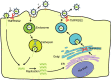
Influenza virus and coronavirus epidemics or pandemics have occurred in succession worldwide throughout the early 21st century. These epidemics or pandemics pose a major threat to human health. Here, we outline a critical role of the host cell protease TMPRSS2 in influenza virus and coronavirus infections and highlight an antiviral therapeutic strategy targeting TMPRSS2.
Keywords: Coronavirus; Influenza virus; TMPRSS2; Therapeutics.
Copyright © 2017 Elsevier B.V. and Société Française de Biochimie et Biologie Moléculaire (SFBBM). All rights reserved.
Figures

Similar articles
-
The Proteolytic Activation of (H3N2) Influenza A Virus Hemagglutinin Is Facilitated by Different Type II Transmembrane Serine Proteases.J Virol. 2016 Apr 14;90(9):4298-4307. doi: 10.1128/JVI.02693-15. Print 2016 May. J Virol. 2016. PMID: 26889029 Free PMC article.
-
Repurposing host-based therapeutics to control coronavirus and influenza virus.Drug Discov Today. 2019 Mar;24(3):726-736. doi: 10.1016/j.drudis.2019.01.018. Epub 2019 Jan 31. Drug Discov Today. 2019. PMID: 30711575 Free PMC article. Review.
-
Airway proteases: an emerging drug target for influenza and other respiratory virus infections.Curr Opin Virol. 2017 Jun;24:16-24. doi: 10.1016/j.coviro.2017.03.018. Epub 2017 Apr 14. Curr Opin Virol. 2017. PMID: 28414992 Free PMC article. Review.
-
Tmprss2 knock-out mice are resistant to H10 influenza A virus pathogenesis.J Gen Virol. 2019 Jul;100(7):1073-1078. doi: 10.1099/jgv.0.001274. Epub 2019 May 17. J Gen Virol. 2019. PMID: 31099738
-
Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation.Biochem J. 2013 Jun 1;452(2):331-43. doi: 10.1042/BJ20130101. Biochem J. 2013. PMID: 23527573
Cited by
-
Interaction between Sars-CoV-2 structural proteins and host cellular receptors: From basic mechanisms to clinical perspectives.Adv Protein Chem Struct Biol. 2022;132:243-277. doi: 10.1016/bs.apcsb.2022.05.010. Epub 2022 Jun 9. Adv Protein Chem Struct Biol. 2022. PMID: 36088078 Free PMC article. Review.
-
ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19.Biomed Pharmacother. 2020 Nov;131:110678. doi: 10.1016/j.biopha.2020.110678. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861070 Free PMC article. Review.
-
Cell-autonomous immune gene expression is repressed in pulmonary neuroendocrine cells and small cell lung cancer.Commun Biol. 2021 Mar 9;4(1):314. doi: 10.1038/s42003-021-01842-7. Commun Biol. 2021. PMID: 33750914 Free PMC article.
-
The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment.Int J Mol Sci. 2022 Jan 25;23(3):1351. doi: 10.3390/ijms23031351. Int J Mol Sci. 2022. PMID: 35163273 Free PMC article. Review.
-
Improving Soluble Expression of SARS-CoV-2 Spike Priming Protease TMPRSS2 with an Artificial Fusing Protein.Int J Mol Sci. 2023 Jun 22;24(13):10475. doi: 10.3390/ijms241310475. Int J Mol Sci. 2023. PMID: 37445653 Free PMC article.
References
-
- Pamuk S. The black death and the origins of the ‘great divergence’ across Europe, 1300–1600. Eur. Rev. Econ. Hist. 2007;11:289–317.
-
- Cohen J. What's old is new: 1918 virus matches 2009 H1N1 strain. Science. 2010;327:1563–1564. - PubMed
-
- Stock I. Yersinia pestis and plague-an update. Med. Monatsschr. Pharm. 2014;37:441–448. - PubMed
-
- Seto W.H., Tsang D., Yung R.W., Ching T.Y., Ng T.K., Ho M., Ho L.M., Peiris J.S. Advisors of Expert SARS group of Hospital Authority, Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

