Production of virus-like particles for vaccines
- PMID: 28778817
- PMCID: PMC7102714
- DOI: 10.1016/j.nbt.2017.07.010
Production of virus-like particles for vaccines
Abstract
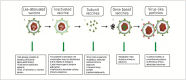
Virus-like particles (VLPs) are nanostructures that resemble the structures of viruses. They are composed of one or more structural proteins that can be arranged in several layers and can also contain a lipid outer envelope. VLPs trigger a high humoral and cellular immune response due to their repetitive structures. A key factor regarding VLP safety is the lack of viral genomic material, which enhances safety during both manufacture and administration. Contemporary VLP production may take advantage of several systems, including bacterial, yeast, insect and mammalian cells. The choice of production platform depends on several factors, including cost and the need for post-translational modifications (PTMs), which can be essential in generating an optimal immune response. Some VLP-based vaccines designed to prevent several infectious diseases are already approved and on the market, with many others at the clinical trial or research stage. Interest in this technology has recently increased due to its advantages over classical vaccines. This paper reviews the state-of-the-art of VLP production systems and the newest generation of VLP-based vaccines now available.
Keywords: Recombinant vaccines; culture mode; immunogen; production platform; virus-like particles.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Plant-derived virus-like particles as vaccines.Hum Vaccin Immunother. 2013 Jan;9(1):26-49. doi: 10.4161/hv.22218. Epub 2012 Sep 20. Hum Vaccin Immunother. 2013. PMID: 22995837 Free PMC article. Review.
-
Recombinant Modified Vaccinia Virus Ankara Generating Ebola Virus-Like Particles.J Virol. 2017 May 12;91(11):e00343-17. doi: 10.1128/JVI.00343-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331098 Free PMC article.
-
Plant-derived VLP: a worthy platform to produce vaccine against SARS-CoV-2.Biotechnol Lett. 2022 Jan;44(1):45-57. doi: 10.1007/s10529-021-03211-0. Epub 2021 Nov 27. Biotechnol Lett. 2022. PMID: 34837582 Free PMC article. Review.
-
Targeted supplementation design for improved production and quality of enveloped viral particles in insect cell-baculovirus expression system.J Biotechnol. 2016 Sep 10;233:34-41. doi: 10.1016/j.jbiotec.2016.06.029. Epub 2016 Jul 1. J Biotechnol. 2016. PMID: 27378622
-
Suitability and perspectives on using recombinant insect cells for the production of virus-like particles.Appl Microbiol Biotechnol. 2014 Mar;98(5):1963-70. doi: 10.1007/s00253-013-5474-9. Epub 2014 Jan 10. Appl Microbiol Biotechnol. 2014. PMID: 24407451 Review.
Cited by
-
Induction of Robust and Specific Humoral and Cellular Immune Responses by Bovine Viral Diarrhea Virus Virus-Like Particles (BVDV-VLPs) Engineered with Baculovirus Expression Vector System.Vaccines (Basel). 2021 Apr 6;9(4):350. doi: 10.3390/vaccines9040350. Vaccines (Basel). 2021. PMID: 33917272 Free PMC article.
-
Evaluation of Virus-Free Manufacture of Recombinant Proteins Using CRISPR-Mediated Gene Disruption in Baculovirus-Infected Insect Cells.Vaccines (Basel). 2023 Jan 19;11(2):225. doi: 10.3390/vaccines11020225. Vaccines (Basel). 2023. PMID: 36851104 Free PMC article.
-
Display of malaria transmission-blocking antigens on chimeric duck hepatitis B virus-derived virus-like particles produced in Hansenula polymorpha.PLoS One. 2019 Sep 4;14(9):e0221394. doi: 10.1371/journal.pone.0221394. eCollection 2019. PLoS One. 2019. PMID: 31483818 Free PMC article.
-
Self-assembled nanoparticles: A new platform for revolutionizing therapeutic cancer vaccines.Front Immunol. 2023 Feb 21;14:1125253. doi: 10.3389/fimmu.2023.1125253. eCollection 2023. Front Immunol. 2023. PMID: 36895553 Free PMC article. Review.
-
Vaccine and vaccination as a part of human life: In view of COVID-19.Biotechnol J. 2022 Jan;17(1):e2100188. doi: 10.1002/biot.202100188. Epub 2021 Nov 21. Biotechnol J. 2022. PMID: 34665927 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous