Discovery of dapivirine, a nonnucleoside HIV-1 reverse transcriptase inhibitor, as a broad-spectrum antiviral against both influenza A and B viruses
- PMID: 28778830
- PMCID: PMC5599177
- DOI: 10.1016/j.antiviral.2017.07.016
Discovery of dapivirine, a nonnucleoside HIV-1 reverse transcriptase inhibitor, as a broad-spectrum antiviral against both influenza A and B viruses
Abstract
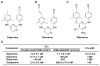
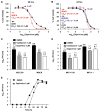
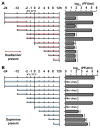
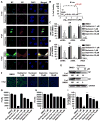
The emergence of multidrug-resistant influenza viruses poses a persistent threat to public health. The current prophylaxis and therapeutic interventions for influenza virus infection have limited efficacy due to the continuous antigenic drift and antigenic shift of influenza viruses. As part of our ongoing effort to develop the next generation of influenza antivirals with broad-spectrum antiviral activity and a high genetic barrier to drug resistance, in this study we report the discovery of dapivirine, an FDA-approved HIV nonnucleoside reverse transcriptase inhibitor, as a broad-spectrum antiviral against multiple strains of influenza A and B viruses with low micromolar efficacy. Mechanistic studies revealed that dapivirine inhibits the nuclear entry of viral ribonucleoproteins at the early stage of viral replication. As a result, viral RNA and protein synthesis were inhibited. Furthermore, dapivirine has a high in vitro genetic barrier to drug resistance, and its antiviral activity is synergistic with oseltamivir carboxylate. In summary, the in vitro antiviral results of dapivirine suggest it is a promising candidate for the development of the next generation of dual influenza and HIV antivirals.
Keywords: Broad-spectrum antiviral; Dapivirine; Drug resistance; Dual antiviral; Influenza virus.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Chemical Genomics Approach Leads to the Identification of Hesperadin, an Aurora B Kinase Inhibitor, as a Broad-Spectrum Influenza Antiviral.Int J Mol Sci. 2017 Sep 8;18(9):1929. doi: 10.3390/ijms18091929. Int J Mol Sci. 2017. PMID: 28885544 Free PMC article.
-
Discovery of cyclosporine A and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance.Antiviral Res. 2016 Sep;133:62-72. doi: 10.1016/j.antiviral.2016.07.019. Epub 2016 Jul 28. Antiviral Res. 2016. PMID: 27478032 Free PMC article.
-
A natural component from Euphorbia humifusa Willd displays novel, broad-spectrum anti-influenza activity by blocking nuclear export of viral ribonucleoprotein.Biochem Biophys Res Commun. 2016 Mar 4;471(2):282-9. doi: 10.1016/j.bbrc.2016.01.123. Epub 2016 Feb 3. Biochem Biophys Res Commun. 2016. PMID: 26850850
-
Influenza A Virus Nucleoprotein: A Highly Conserved Multi-Functional Viral Protein as a Hot Antiviral Drug Target.Curr Top Med Chem. 2017;17(20):2271-2285. doi: 10.2174/1568026617666170224122508. Curr Top Med Chem. 2017. PMID: 28240183 Free PMC article. Review.
-
Influenza drug resistance.Semin Respir Crit Care Med. 2011 Aug;32(4):409-22. doi: 10.1055/s-0031-1283281. Epub 2011 Aug 19. Semin Respir Crit Care Med. 2011. PMID: 21858746 Review.
Cited by
-
A Novel Capsid Binding Inhibitor Displays Potent Antiviral Activity against Enterovirus D68.ACS Infect Dis. 2019 Nov 8;5(11):1952-1962. doi: 10.1021/acsinfecdis.9b00284. Epub 2019 Sep 18. ACS Infect Dis. 2019. PMID: 31532189 Free PMC article.
-
A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection.Sci Data. 2021 Feb 26;8(1):70. doi: 10.1038/s41597-021-00848-4. Sci Data. 2021. PMID: 33637768 Free PMC article.
-
Overview of Viral Pneumonia Associated With Influenza Virus, Respiratory Syncytial Virus, and Coronavirus, and Therapeutics Based on Natural Products of Medicinal Plants.Front Pharmacol. 2021 Jun 21;12:630834. doi: 10.3389/fphar.2021.630834. eCollection 2021. Front Pharmacol. 2021. PMID: 34234668 Free PMC article. Review.
-
Drug Repurposing Approaches for the Treatment of Influenza Viral Infection: Reviving Old Drugs to Fight Against a Long-Lived Enemy.Front Immunol. 2019 Mar 19;10:531. doi: 10.3389/fimmu.2019.00531. eCollection 2019. Front Immunol. 2019. PMID: 30941148 Free PMC article. Review.
-
Boceprevir, Calpain Inhibitors II and XII, and GC-376 Have Broad-Spectrum Antiviral Activity against Coronaviruses.ACS Infect Dis. 2021 Mar 12;7(3):586-597. doi: 10.1021/acsinfecdis.0c00761. Epub 2021 Mar 1. ACS Infect Dis. 2021. PMID: 33645977 Free PMC article.
References
-
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. - PubMed
-
- Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, Team MAS. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375:2121–2132. - PMC - PubMed
-
- Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41:93–141. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

