A Web-Based Public Health Intervention to Reduce Functional Impairment and Depressive Symptoms in Adults With Type 2 Diabetes (The SpringboarD Trial): Randomized Controlled Trial Protocol
- PMID: 28778848
- PMCID: PMC5561386
- DOI: 10.2196/resprot.7348
A Web-Based Public Health Intervention to Reduce Functional Impairment and Depressive Symptoms in Adults With Type 2 Diabetes (The SpringboarD Trial): Randomized Controlled Trial Protocol
Abstract
Background: Depressive symptoms are common in people with type 2 diabetes and contribute to adverse health consequences that substantially impact social and vocational function. Despite the existence of effective depression treatments, the majority of people with type 2 diabetes do not access these when needed. Web-based alternatives to more traditional psychotherapies offer a potential solution to reducing the personal and economic burdens of type 2 diabetes.
Objective: This paper outlines the protocol for a randomized controlled trial (RCT) of myCompass, a Web-based public health psychotherapy intervention, in people with type 2 diabetes. Fully automated, interactive, and delivered via the Internet without clinician support, myCompass teaches cognitive behavioral therapy-based skills and supports symptom monitoring to improve daily functioning and reduce mild-to-moderate mental health symptoms.
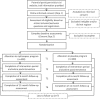
Methods: A two-arm RCT will be conducted. People with type 2 diabetes and mild-to-moderately severe depressive symptoms will be recruited from the community and general practice settings. Screening and enrollment is via an open-access website. Participants will be randomized to use either myCompass or an active placebo program for 8 weeks, followed by a 4-week tailing-off period. The placebo program is matched to myCompass on mode of delivery, interactivity, and duration. Outcomes will be assessed at baseline and at 3-month, 6-month, and 12-month follow-up. The primary study outcome is work and social functioning. Secondary study outcomes include depressive and anxious symptoms, diabetes-related distress, self-care behaviors, and glycemic control.
Results: Nationwide recruitment is currently underway with the aim of recruiting 600 people with type 2 diabetes. Recruitment will continue until October 2017.
Conclusions: This is the first known trial of a Web-based psychotherapy program that is not diabetes specific for improving social and vocational function in people with type 2 diabetes and mild-to-moderately severe depressive symptoms. With the increasing prevalence of type 2 diabetes and depression, a potentially scalable public health intervention could play a very large role in reducing unmet mental health need and ameliorating the personal and societal impact of illness comorbidity.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR) Number: ACTRN12615000931572; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368109 (Archived by WebCite at http://www.webcitation.org/ 6rh3imVMh).
Keywords: Web-based intervention; depression; type 2 diabetes.
©Judy Proudfoot, Janine Clarke, Jane Gunn, Susan Fletcher, Samineh Sanatkar, Kay Wilhelm, Lesley Campbell, Nicholas Zwar, Mark Harris, Helen Lapsley, Dusan Hadzi-Pavlovic, Helen Christensen. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 03.08.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008 Nov;121(11 Suppl 2):S8–S15. doi: 10.1016/j.amjmed.2008.09.008. http://europepmc.org/abstract/MED/18954592 - DOI - PMC - PubMed
-
- Egede LE. Effects of depression on work loss and disability bed days in individuals with diabetes. Diabetes Care. 2004 Jul;27(7):1751–1753. - PubMed
-
- Manigault KR. The bidirectional relationship between depression and diabetes. US Pharm. 2016 Nov 17;41(11):26–29. https://www.uspharmacist.com/article/the-bidirectional-relationship-betw...
LinkOut - more resources
Full Text Sources
Other Literature Sources

