Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD
- PMID: 28778860
- PMCID: PMC5619975
- DOI: 10.1681/ASN.2017030247
Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD
Abstract
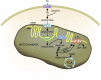
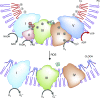
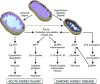
AKI is associated with high morbidity and mortality, and it predisposes to the development and progression of CKD. Novel strategies that minimize AKI and halt the progression of CKD are urgently needed. Normal kidney function involves numerous different cell types, such as tubular epithelial cells, endothelial cells, and podocytes, working in concert. This delicate balance involves many energy-intensive processes. Fatty acids are the preferred energy substrates for the kidney, and defects in fatty acid oxidation and mitochondrial dysfunction are universally involved in diverse causes of AKI and CKD. This review provides an overview of ATP production and energy demands in the kidney and summarizes preclinical and clinical evidence of mitochondrial dysfunction in AKI and CKD. New therapeutic strategies targeting mitochondria protection and cellular bioenergetics are presented, with emphasis on those that have been evaluated in animal models of AKI and CKD. Targeting mitochondrial function and cellular bioenergetics upstream of cellular damage may offer advantages compared with targeting downstream inflammatory and fibrosis processes.
Keywords: diabetic nephropathy; fibrosis; ischemia-reperfusion; metabolism; mitochondria; reactive oxygen species.
Copyright © 2017 by the American Society of Nephrology.
Figures
Similar articles
-
The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential.Int J Mol Sci. 2021 Oct 19;22(20):11253. doi: 10.3390/ijms222011253. Int J Mol Sci. 2021. PMID: 34681922 Free PMC article. Review.
-
Mitochondrial dysfunction and the AKI-to-CKD transition.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F1105-F1116. doi: 10.1152/ajprenal.00285.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073587 Review.
-
Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis.Am J Physiol Renal Physiol. 2014 May 1;306(9):F970-80. doi: 10.1152/ajprenal.00697.2013. Epub 2014 Feb 19. Am J Physiol Renal Physiol. 2014. PMID: 24553434
-
Chronic impairment of mitochondrial bioenergetics and β-oxidation promotes experimental AKI-to-CKD transition induced by folic acid.Free Radic Biol Med. 2020 Jul;154:18-32. doi: 10.1016/j.freeradbiomed.2020.04.016. Epub 2020 Apr 30. Free Radic Biol Med. 2020. PMID: 32360615
-
Mitochondrial Signaling, the Mechanisms of AKI-to-CKD Transition and Potential Treatment Targets.Int J Mol Sci. 2024 Jan 26;25(3):1518. doi: 10.3390/ijms25031518. Int J Mol Sci. 2024. PMID: 38338797 Free PMC article. Review.
Cited by
-
Mitochondrial destabilization in tendinopathy and potential therapeutic strategies.J Orthop Translat. 2024 Oct 3;49:49-61. doi: 10.1016/j.jot.2024.09.003. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 39430132 Free PMC article. Review.
-
Defining the short-term effects of pharmacological 5'-AMP activated kinase modulators on mitochondrial polarization, morphology and heterogeneity.PeerJ. 2018 Aug 30;6:e5469. doi: 10.7717/peerj.5469. eCollection 2018. PeerJ. 2018. PMID: 30186684 Free PMC article.
-
Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells.Exp Mol Med. 2019 Jul 9;51(7):1-14. doi: 10.1038/s12276-019-0268-5. Exp Mol Med. 2019. PMID: 31285429 Free PMC article.
-
Mitochondrial dysfunction and oxidative stress are involved in the mechanism of tramadol-induced renal injury.Curr Res Pharmacol Drug Discov. 2021 Sep 3;2:100049. doi: 10.1016/j.crphar.2021.100049. eCollection 2021. Curr Res Pharmacol Drug Discov. 2021. PMID: 34909675 Free PMC article.
-
Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver.Antioxidants (Basel). 2022 Nov 6;11(11):2195. doi: 10.3390/antiox11112195. Antioxidants (Basel). 2022. PMID: 36358567 Free PMC article.
References
-
- Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 - PubMed
-
- Mount PF, Power DA: Balancing the energy equation for healthy kidneys. J Pathol 237: 407–410, 2015 - PubMed
-
- Houten SM, Violante S, Ventura FV, Wanders RJ: The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu Rev Physiol 78: 23–44, 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

