Ticks from diverse genera encode chemokine-inhibitory evasin proteins
- PMID: 28778927
- PMCID: PMC5612101
- DOI: 10.1074/jbc.M117.807255
Ticks from diverse genera encode chemokine-inhibitory evasin proteins
Erratum in
-
Correction: Ticks from diverse genera encode chemokine-inhibitory evasin proteins.J Biol Chem. 2018 Oct 12;293(41):15888. doi: 10.1074/jbc.AAC118.005871. J Biol Chem. 2018. PMID: 30315086 Free PMC article. No abstract available.
Abstract
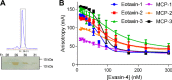
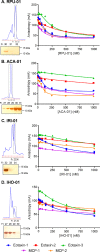
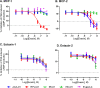
To prolong residence on their hosts, ticks secrete many salivary factors that target host defense molecules. In particular, the tick Rhipicephalus sanguineus has been shown to produce three salivary glycoproteins named "evasins," which bind to host chemokines, thereby inhibiting the recruitment of leukocytes to the location of the tick bite. Using sequence similarity searches, we have identified 257 new putative evasin sequences encoded by the genomes or salivary or visceral transcriptomes of numerous hard ticks, spanning the genera Rhipicephalus, Amblyomma, and Ixodes of the Ixodidae family. Nine representative sequences were successfully expressed in Escherichia coli, and eight of the nine candidates exhibited high-affinity binding to human chemokines. Sequence alignments enabled classification of the evasins into two subfamilies: C8 evasins share a conserved set of eight Cys residues (four disulfide bonds), whereas C6 evasins have only three of these disulfide bonds. Most of the identified sequences contain predicted secretion leader sequences, N-linked glycosylation sites, and a putative site of tyrosine sulfation. We conclude that chemokine-binding evasin proteins are widely expressed among tick species of the Ixodidae family, are likely to play important roles in subverting host defenses, and constitute a valuable pool of anti-inflammatory proteins for potential future therapeutic applications.
Keywords: bioinformatics; chemokine; evasin; fluorescence anisotropy; host-pathogen interaction; inhibitor; recombinant protein expression; tick protein.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Phylogenetic Analysis Indicates That Evasin-Like Proteins of Ixodid Ticks Fall Into Three Distinct Classes.Front Cell Infect Microbiol. 2021 Oct 22;11:769542. doi: 10.3389/fcimb.2021.769542. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34746035 Free PMC article.
-
Evasins: Tick Salivary Proteins that Inhibit Mammalian Chemokines.Trends Biochem Sci. 2020 Feb;45(2):108-122. doi: 10.1016/j.tibs.2019.10.003. Epub 2019 Nov 1. Trends Biochem Sci. 2020. PMID: 31679840 Free PMC article. Review.
-
The N-terminal domain of a tick evasin is critical for chemokine binding and neutralization and confers specific binding activity to other evasins.J Biol Chem. 2018 Apr 20;293(16):6134-6146. doi: 10.1074/jbc.RA117.000487. Epub 2018 Feb 27. J Biol Chem. 2018. PMID: 29487134 Free PMC article.
-
Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus.J Biol Chem. 2007 Sep 14;282(37):27250-27258. doi: 10.1074/jbc.M704706200. Epub 2007 Jul 19. J Biol Chem. 2007. PMID: 17640866
-
Using evasins to target the chemokine network in inflammation.Adv Protein Chem Struct Biol. 2020;119:1-38. doi: 10.1016/bs.apcsb.2019.09.003. Epub 2019 Nov 26. Adv Protein Chem Struct Biol. 2020. PMID: 31997766 Review.
Cited by
-
Influence of Chemokine N-Terminal Modification on Biased Agonism at the Chemokine Receptor CCR1.Int J Mol Sci. 2019 May 15;20(10):2417. doi: 10.3390/ijms20102417. Int J Mol Sci. 2019. PMID: 31096719 Free PMC article.
-
Time-resolved proteomic profile of Amblyomma americanum tick saliva during feeding.PLoS Negl Trop Dis. 2020 Feb 12;14(2):e0007758. doi: 10.1371/journal.pntd.0007758. eCollection 2020 Feb. PLoS Negl Trop Dis. 2020. PMID: 32049966 Free PMC article.
-
Probing the Rhipicephalusbursa Sialomes in Potential Anti-Tick Vaccine Candidates: A Reverse Vaccinology Approach.Biomedicines. 2021 Mar 31;9(4):363. doi: 10.3390/biomedicines9040363. Biomedicines. 2021. PMID: 33807386 Free PMC article.
-
Salp14 epitope-based mRNA vaccination induces early recognition of a tick bite.Vaccine. 2024 Oct 24;42(24):126304. doi: 10.1016/j.vaccine.2024.126304. Epub 2024 Sep 5. Vaccine. 2024. PMID: 39236403
-
Chemical Equilibrium at the Tick-Host Feeding Interface:A Critical Examination of Biological Relevance in Hematophagous Behavior.Front Physiol. 2019 May 1;10:530. doi: 10.3389/fphys.2019.00530. eCollection 2019. Front Physiol. 2019. PMID: 31118903 Free PMC article. Review.
References
-
- Dantas-Torres F., Chomel B. B., and Otranto D. (2012) Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 28, 437–446 - PubMed
-
- de la Fuente J., Ayoubi P., Blouin E. F., Almazán C., Naranjo V., and Kocan K. M. (2006) Anaplasmosis: focusing on host–vector–pathogen interactions for vaccine development. Ann. N.Y. Acad. Sci. 1078, 416–423 - PubMed
-
- Fivaz B., Petney T., and Horak I. (1992) Tick Vector Biology, Springer-Verlag, Berlin
-
- Moser B., Wolf M., Walz A., and Loetscher P. (2004) Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 25, 75–84 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

