Disturbed Flow Promotes Arterial Stiffening Through Thrombospondin-1
- PMID: 28778947
- PMCID: PMC5614852
- DOI: 10.1161/CIRCULATIONAHA.116.026361
Disturbed Flow Promotes Arterial Stiffening Through Thrombospondin-1
Abstract
Background: Arterial stiffness and wall shear stress are powerful determinants of cardiovascular health, and arterial stiffness is associated with increased cardiovascular mortality. Low and oscillatory wall shear stress, termed disturbed flow (d-flow), promotes atherosclerotic arterial remodeling, but the relationship between d-flow and arterial stiffness is not well understood. The objective of this study was to define the role of d-flow on arterial stiffening and discover the relevant signaling pathways by which d-flow stiffens arteries.
Methods: D-flow was induced in the carotid arteries of young and old mice of both sexes. Arterial stiffness was quantified ex vivo with cylindrical biaxial mechanical testing and in vivo from duplex ultrasound and compared with unmanipulated carotid arteries from 80-week-old mice. Gene expression and pathway analysis was performed on endothelial cell-enriched RNA and validated by immunohistochemistry. In vitro testing of signaling pathways was performed under oscillatory and laminar wall shear stress conditions. Human arteries from regions of d-flow and stable flow were tested ex vivo to validate critical results from the animal model.
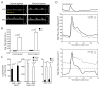
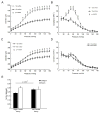
Results: D-flow induced arterial stiffening through collagen deposition after partial carotid ligation, and the degree of stiffening was similar to that of unmanipulated carotid arteries from 80-week-old mice. Intimal gene pathway analyses identified transforming growth factor-β pathways as having a prominent role in this stiffened arterial response, but this was attributable to thrombospondin-1 (TSP-1) stimulation of profibrotic genes and not changes to transforming growth factor-β. In vitro and in vivo testing under d-flow conditions identified a possible role for TSP-1 activation of transforming growth factor-β in the upregulation of these genes. TSP-1 knockout animals had significantly less arterial stiffening in response to d-flow than wild-type carotid arteries. Human arteries exposed to d-flow had similar increases TSP-1 and collagen gene expression as seen in our model.
Conclusions: TSP-1 has a critical role in shear-mediated arterial stiffening that is mediated in part through TSP-1's activation of the profibrotic signaling pathways of transforming growth factor-β. Molecular targets in this pathway may lead to novel therapies to limit arterial stiffening and the progression of disease in arteries exposed to d-flow.
Keywords: disturbed flow; thrombospondins; vascular stiffness.
© 2017 American Heart Association, Inc.
Figures


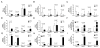



Similar articles
-
Endothelial cell (EC)-specific Ctgf/Ccn2 expression increases EC reprogramming and atherosclerosis.Matrix Biol. 2025 Apr;136:102-110. doi: 10.1016/j.matbio.2025.01.003. Epub 2025 Jan 14. Matrix Biol. 2025. PMID: 39818254
-
Modulation of arterial intima stiffness by disturbed blood flow.Exp Biol Med (Maywood). 2024 Jul 31;249:10090. doi: 10.3389/ebm.2024.10090. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39143955 Free PMC article.
-
Disruption of TGF-β signaling in smooth muscle cell prevents flow-induced vascular remodeling.Biochem Biophys Res Commun. 2014 Nov 7;454(1):245-50. doi: 10.1016/j.bbrc.2014.10.092. Epub 2014 Oct 24. Biochem Biophys Res Commun. 2014. PMID: 25451249
-
Lifelong Cyclic Mechanical Strain Promotes Large Elastic Artery Stiffening: Increased Pulse Pressure and Old Age-Related Organ Failure.Can J Cardiol. 2016 May;32(5):624-33. doi: 10.1016/j.cjca.2015.12.022. Epub 2015 Dec 29. Can J Cardiol. 2016. PMID: 26961664 Review.
-
Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease.Circ Res. 2015 Feb 27;116(5):895-908. doi: 10.1161/CIRCRESAHA.116.305720. Circ Res. 2015. PMID: 25722443 Review.
Cited by
-
Thrombospondin 1 in Metabolic Diseases.Front Endocrinol (Lausanne). 2021 Mar 29;12:638536. doi: 10.3389/fendo.2021.638536. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33854480 Free PMC article. Review.
-
Matricellular proteins in atherosclerosis development.Matrix Biol. 2023 Jun;120:1-23. doi: 10.1016/j.matbio.2023.04.003. Epub 2023 Apr 20. Matrix Biol. 2023. PMID: 37086928 Free PMC article. Review.
-
Flow-induced reprogramming of endothelial cells in atherosclerosis.Nat Rev Cardiol. 2023 Nov;20(11):738-753. doi: 10.1038/s41569-023-00883-1. Epub 2023 May 24. Nat Rev Cardiol. 2023. PMID: 37225873 Free PMC article. Review.
-
Transcriptional drifts associated with environmental changes in endothelial cells.Elife. 2023 Mar 27;12:e81370. doi: 10.7554/eLife.81370. Elife. 2023. PMID: 36971339 Free PMC article.
-
Thrombospondin-1 in vascular development, vascular function, and vascular disease.Semin Cell Dev Biol. 2024 Mar 1;155(Pt B):32-44. doi: 10.1016/j.semcdb.2023.07.011. Epub 2023 Jul 27. Semin Cell Dev Biol. 2024. PMID: 37507331 Free PMC article. Review.
References
-
- Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 2015;116:895–908. - PubMed
-
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. - PubMed
-
- London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. - PubMed
-
- Rammos C, Hendgen-Cotta UB, Deenen R, Pohl J, Stock P, Hinzmann C, Kelm M, Rassaf T. Age-related vascular gene expression profiling in mice. Mech Ageing Dev. 2014;135:15–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

