Cell-Free DNA in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis
- PMID: 28778958
- PMCID: PMC5599199
- DOI: 10.1634/theoncologist.2016-0178
Cell-Free DNA in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis
Abstract
Background: Circulating DNA can be detected and quantified in the blood of cancer patients and used for detection of tumor-specific genetic alterations. The clinical utility has been intensively investigated for the past 10 years. The majority of reports focus on analyzing the clinical potential of tumor-specific mutations, whereas the use of total cell-free DNA (cfDNA) quantification is somehow controversial and sparsely described in the literature, but holds important clinical information in itself. The purpose of the present report was to present a systematic review and meta-analysis of the prognostic value of total cfDNA in patients with metastatic colorectal cancer (mCRC) treated with chemotherapy. In addition, we report on the overall performance of cfDNA as source for KRAS mutation detection.
Materials and methods: A systematic literature search of PubMed and Embase was performed by two independent investigators. Eligibility criteria were (a) total cfDNA analysis, (b) mCRC, and (c) prognostic value during palliative treatment. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were followed, and meta-analysis applied on both aggregate data extraction and individual patients' data.
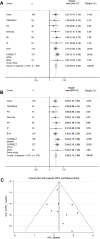
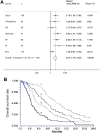
Results: Ten eligible cohorts were identified, including a total of 1,076 patients. Seven studies used quantitative polymerase chain reaction methods, two BEAMing [beads, emulsification, amplification, and magnetics] technology, and one study digital droplet polymerase chain reaction. The baseline levels of cfDNA was similar in the presented studies, and all studies reported a clear prognostic value in favor of patients with lowest levels of baseline cfDNA. A meta-analysis revealed a combined estimate of favorable overall survival hazard ratio (HR) in patients with levels below the median cfDNA (HR = 2.39, 95% confidence interval 2.03-2.82, p < .0001).
Conclusion: The total cfDNA levels are high in patients with mCRC and bear strong prognostic information, which should be tested prospectively by using a predefined cut-off value based on normal values in healthy cohorts. Finally, the potential use of cfDNA for detection of tumor-specific mutations was emphasized in a large individual patients' data meta-analysis.
Implications for practice: Reliable prognostic markers could help to guide patients and treating physicians regarding the relevance and choice of systemic therapy. Small fragments of circulating cell-free DNA (cfDNA) can be measured in a simple blood sample. This report presents the first meta-analysis of the prognostic value of total cfDNA measurement in patients with metastatic colorectal cancer. Data from 1,076 patients confirmed that patients with the lowest pre-treatment levels of cfDNA had a significantly higher chance of longer survival than those with higher levels. Cell-free DNA analysis can also be used for detection of tumor-specific mutations, and hold potential as a valuable tool in colorectal cancer treatment.
Keywords: Cell‐free DNA plasma; Metastatic colorectal cancer; Meta‐analysis; Prognosis.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Cunningham D, Atkin W, Lenz HJ et al. Colorectal cancer. Lancet 2010;375:1030–1047. - PubMed
-
- Therkildsen C, Bergmann TK, Henrichsen‐Schnack T et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti‐EGFR treatment in metastatic colorectal cancer: A systematic review and meta‐analysis. Acta Oncol 2014;53:852–864. - PubMed
-
- Jahr S, Hentze H, Englisch S et al. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659–1665. - PubMed
-
- Leon SA, Shapiro B, Sklaroff DM et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1997;37:646–650. - PubMed
-
- De Mattos‐Arruda L, Olmos D, Tabernero J. Prognostic and predictive roles for circulating biomarkers in gastrointestinal cancer. Future Oncol 2011;7:1385–1397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

