Regulation of Energy Metabolism by Bone-Derived Hormones
- PMID: 28778968
- PMCID: PMC5983159
- DOI: 10.1101/cshperspect.a031666
Regulation of Energy Metabolism by Bone-Derived Hormones
Abstract
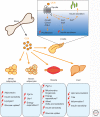
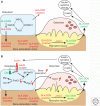
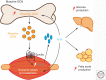
Like many other organs, bone can act as an endocrine organ through the secretion of bone-specific hormones or "osteokines." At least two osteokines are implicated in the control of glucose and energy metabolism: osteocalcin (OCN) and lipocalin-2 (LCN2). OCN stimulates the production and secretion of insulin by the pancreatic β-cells, but also favors adaptation to exercise by stimulating glucose and fatty acid (FA) utilization by the muscle. Both of these OCN functions are mediated by the G-protein-coupled receptor GPRC6A. In contrast, LCN2 influences energy metabolism by activating appetite-suppressing signaling in the brain. This action of LCN2 occurs through its binding to the melanocortin 4 receptor (MC4R) in the paraventricular nucleus of the hypothalamus (PVN) and ventromedial neurons of the hypothalamus.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Bonneau J, Ferland G, Karelis AD, Doucet E, Faraj M, Rabasa-Lhoret R, Ferron M. 2017. Association between osteocalcin γ-carboxylation and insulin resistance in overweight and obese postmenopausal women. J Diabetes Complications 31: 1027–1034. - PubMed
-
- Borges BC, Rorato R, Avraham Y, da Silva LE, Castro M, Vorobiav L, Berry E, Antunes-Rodrigues J, Elias LL. 2011. Leptin resistance and desensitization of hypophagia during prolonged inflammatory challenge. Am J Physiol Endocrinol Metab 300: E858–E869. - PubMed
-
- Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, et al. 2002. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175: 405–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
