Impact of folic acid intake during pregnancy on genomic imprinting of IGF2/H19 and 1-carbon metabolism
- PMID: 28778973
- PMCID: PMC5690385
- DOI: 10.1096/fj.201601214RR
Impact of folic acid intake during pregnancy on genomic imprinting of IGF2/H19 and 1-carbon metabolism
Abstract
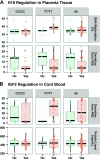
Folic acid is an essential component of 1-carbon metabolism, which generates methyl groups for DNA methylation. Disruption of genomic imprinting leads to biallelic expression which may affect disease susceptibility possibly reflected in high levels of S-adenosyl-homocysteine (SAH) and low levels of S-adenosyl-methionine (SAM). We investigated the association between folic acid supplementation during pregnancy and loss of imprinting (LOI) of IGF2 and H19 genes in placentas and cord blood of 90 mother-child dyads in association with the methylenetetrahydrofolate reductase (MTHFR) genotype. Pyrosequencing was used to evaluate deviation from monoallelic expression among 47 placentas heterozygous for H19 and 37 placentas and cord blood tissues heterozygous for IGF2 and H19 methylation levels of 48 placentas. We detected relaxation of imprinting (ROI) and LOI of H19 in placentas not associated with differences in methylation levels of the H19ICR. Placentas retained monoallelic allele-specific gene expression of IGF2, but 32.4% of cord blood samples displayed LOI of IGF2 and 10.8% showed ROI. High SAH levels were significantly associated with low H19 methylation. An interesting positive association between SAM/SAH ratio and high H19 methylation levels was detected among infants with low B12 levels. Our data suggest profound differences in regulation of imprinting in placenta and cord blood; a lack of correlation of the methylome, transcriptome, and proteome; and a complex regulatory feedback network between free methyl groups and genomic imprinting at birth.-Tserga, A., Binder, A. M., Michels, K. B. Impact of folic acid intake during pregnancy on genomic imprinting of IGF2/H19 and 1-carbon metabolism.
Keywords: DNA methylation; MTHFR; epigenetics; folate; placenta.
© FASEB.
Figures


Similar articles
-
Quantitative allele-specific expression and DNA methylation analysis of H19, IGF2 and IGF2R in the human placenta across gestation reveals H19 imprinting plasticity.PLoS One. 2012;7(12):e51210. doi: 10.1371/journal.pone.0051210. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227253 Free PMC article.
-
Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction.Epigenetics. 2010 May 16;5(4):313-24. doi: 10.4161/epi.5.4.11637. Epub 2010 May 28. Epigenetics. 2010. PMID: 20418667
-
Altered dietary ratio of folic acid and vitamin B12 during pregnancy influences the expression of imprinted H19/IGF2 locus in C57BL/6 mice.Br J Nutr. 2022 Oct 28;128(8):1470-1489. doi: 10.1017/S0007114521004220. Epub 2021 Oct 20. Br J Nutr. 2022. PMID: 34666844
-
Genomic imprinting in primate embryos and embryonic stem cells.Reprod Fertil Dev. 2006;18(8):817-21. doi: 10.1071/rd06112. Reprod Fertil Dev. 2006. PMID: 17147929 Review.
-
Association between maternal diet, smoking, and the placenta MTHFR 677C/T genotype and global placental DNA methylation.Placenta. 2024 Feb;146:17-24. doi: 10.1016/j.placenta.2023.12.017. Epub 2023 Dec 19. Placenta. 2024. PMID: 38160599 Review.
Cited by
-
Functional polymorphisms of the lncRNA H19 promoter region contribute to the cancer risk and clinical outcomes in advanced colorectal cancer.Cancer Cell Int. 2019 Aug 20;19:215. doi: 10.1186/s12935-019-0895-x. eCollection 2019. Cancer Cell Int. 2019. PMID: 31452627 Free PMC article.
-
Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women.Nutrients. 2024 Jun 4;16(11):1765. doi: 10.3390/nu16111765. Nutrients. 2024. PMID: 38892698 Free PMC article.
-
The DNA demethylase TET1 modifies the impact of maternal folic acid status on embryonic brain development.EMBO Rep. 2025 Jan;26(1):175-199. doi: 10.1038/s44319-024-00316-1. Epub 2024 Nov 22. EMBO Rep. 2025. PMID: 39578553 Free PMC article.
-
The one-carbon metabolism as an underlying pathway for placental DNA methylation - a systematic review.Epigenetics. 2024 Dec;19(1):2318516. doi: 10.1080/15592294.2024.2318516. Epub 2024 Mar 14. Epigenetics. 2024. PMID: 38484284 Free PMC article.
-
LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells.J Exp Clin Cancer Res. 2020 Jul 11;39(1):131. doi: 10.1186/s13046-020-01610-1. J Exp Clin Cancer Res. 2020. PMID: 32653028 Free PMC article.
References
-
- Laanpere M., Altmäe S., Stavreus-Evers A., Nilsson T. K., Yngve A., Salumets A. (2010) Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viability. Nutr. Rev. 68, 99–113 - PubMed
-
- Bailey L. B., Stover P. J., McNulty H., Fenech M. F., Gregory J. F. III, Mills J. L., Pfeiffer C. M., Fazili Z., Zhang M., Ueland P. M., Molloy A. M., Caudill M. A., Shane B., Berry R. J., Bailey R. L., Hausman D. B., Raghavan R., Raiten D. J. (2015) Biomarkers of nutrition for development-folate review. J. Nutr. 145, 1636S–1680S - PMC - PubMed
-
- Czeizel A. E., Dudás I. (1992) Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 327, 1832–1835 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

