Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux
- PMID: 28779013
- PMCID: PMC5577177
- DOI: 10.1042/BSR20170934
Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux
Abstract
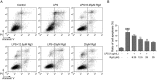
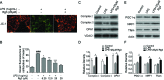
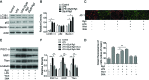
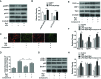
Sepsis-led mitochondrial dysfunction has become a critical pathophysiological procedure in sepsis. Since ginsenosides have been applied in the treatment of mitochondrial dysfunction, ginsenoside Rg3 was employed to study its effects on the mitochondrial dysfunction induced by sepsis. The apoptosis rate, oxygen consumption rate (OCR), reactive oxygen species (ROS), antioxidant glutathione (GSH) pools, and mitochondrial transmembrane potential (MTP) were determined in LPS-induced sepsis hepatocytes treated with different concentrations of Rg3. Then, the protein expression levels of mitochondrial biogenesis related transcription factors, autophagy-related proteins, and AMP-activated protein kinase (AMPK) signal pathway related proteins were determined by Western blotting in both in vitro and in vivo sepsis models. Rg3 shows functions of promotion of OCR, attenuation of ROS, and maintenance of GSH pools, and its conjugating activity in the in vitro sepsis models. Rg3-treated cells were observed to have a higher MTP value compared with the LPS only induced cells. Moreover, Rg3 treatment can inhibit mitochondrial dysfunction via increasing the protein expression levels of mitochondrial biogenesis related transcription factors. Rg3 treatment has the function of inhibitor of apoptosis of human primary hepatocytes, and Rg3 can up-regulate the autophagy-related proteins and activate AMPK signal pathway in sepsis models. Meanwhile, the mitochondrial protective function exerted by Rg3 decreased after the autophagy inhibitors or AMPK inhibitor treatment in LPS-induced human primary hepatocytes. Rg3 can improve mitochondrial dysfunction by regulating autophagy in mitochondria via activating the AMPK signal pathway, thus protecting cell and organ injuries caused by sepsis.
Keywords: Autophagy; Liver injury; Mitochondrial dysfunction; Rg3; Sepsis.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Ginsenoside Rg3 alleviates septic liver injury by regulating the lncRNA TUG1/miR-200c-3p/SIRT1 axis.J Inflamm (Lond). 2021 Dec 20;18(1):31. doi: 10.1186/s12950-021-00296-2. J Inflamm (Lond). 2021. Retraction in: J Inflamm (Lond). 2025 Feb 18;22(1):10. doi: 10.1186/s12950-025-00435-z. PMID: 34930287 Free PMC article. Retracted.
-
Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis via AMPK/mTOR signaling pathway.Drug Dev Res. 2020 Nov;81(7):875-884. doi: 10.1002/ddr.21705. Epub 2020 Jun 17. Drug Dev Res. 2020. PMID: 32898934
-
Corosolic acid protects hepatocytes against ethanol-induced damage by modulating mitogen-activated protein kinases and activating autophagy.Eur J Pharmacol. 2016 Nov 15;791:578-588. doi: 10.1016/j.ejphar.2016.09.031. Epub 2016 Sep 20. Eur J Pharmacol. 2016. PMID: 27663281
-
Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress.Biochim Biophys Acta. 2014 Apr;1840(4):1331-44. doi: 10.1016/j.bbagen.2013.10.034. Epub 2013 Oct 27. Biochim Biophys Acta. 2014. PMID: 24513455 Review.
-
[Research progress in AMP-activated protein kinase and sepsis-induced cardiac dysfunction].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018 Dec 28;43(12):1364-1368. doi: 10.11817/j.issn.1672-7347.2018.12.013. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30643054 Review. Chinese.
Cited by
-
Shenfu injection attenuates cardiac dysfunction and inhibits apoptosis in septic mice.Ann Transl Med. 2022 May;10(10):597. doi: 10.21037/atm-22-836. Ann Transl Med. 2022. PMID: 35722372 Free PMC article.
-
Proteomic analyses reveal that ginsenoside Rg3(S) partially reverses cellular senescence in human dermal fibroblasts by inducing peroxiredoxin.J Ginseng Res. 2020 Jan;44(1):50-57. doi: 10.1016/j.jgr.2018.07.008. Epub 2018 Aug 13. J Ginseng Res. 2020. PMID: 32148389 Free PMC article.
-
Mechanism of Mitophagy and Its Role in Sepsis Induced Organ Dysfunction: A Review.Front Cell Dev Biol. 2021 Jun 7;9:664896. doi: 10.3389/fcell.2021.664896. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34164394 Free PMC article. Review.
-
Ginsenoside Rg3 alleviates septic liver injury by regulating the lncRNA TUG1/miR-200c-3p/SIRT1 axis.J Inflamm (Lond). 2021 Dec 20;18(1):31. doi: 10.1186/s12950-021-00296-2. J Inflamm (Lond). 2021. Retraction in: J Inflamm (Lond). 2025 Feb 18;22(1):10. doi: 10.1186/s12950-025-00435-z. PMID: 34930287 Free PMC article. Retracted.
-
Ginsenoside Rh2 Alleviate Sepsis-related Encephalopathy via Up-regulating Nrf2/HO-1 Pathway and Apoptosis Inhibition.Cell Biochem Biophys. 2025 Mar;83(1):587-597. doi: 10.1007/s12013-024-01488-2. Epub 2024 Aug 26. Cell Biochem Biophys. 2025. PMID: 39187743
References
-
- Levy M.M., Dellinger R.P., Townsend S.R., Linde-Zwirble W.T., Marshall J.C., Bion J. et al. (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit. Care Med. 38, 367–374 - PubMed
-
- Kallinen O., Maisniemi K., Bohling T., Tukiainen E. and Koljonen V. (2012) Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 33, 206–211 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

