Pathogenicity and peramivir efficacy in immunocompromised murine models of influenza B virus infection
- PMID: 28779075
- PMCID: PMC5544712
- DOI: 10.1038/s41598-017-07433-z
Pathogenicity and peramivir efficacy in immunocompromised murine models of influenza B virus infection
Abstract
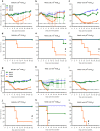
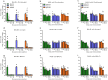
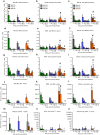
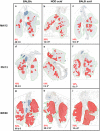
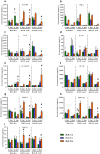
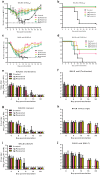
Influenza B viruses are important human pathogens that remain inadequately studied, largely because available animal models are poorly defined. Here, we developed an immunocompromised murine models for influenza B virus infection, which we subsequently used to study pathogenicity and to examine antiviral efficacy of the neuraminidase inhibitor peramivir. We studied three influenza B viruses that represent both the Yamagata (B/Massachusetts/2/2012 and B/Phuket/3073/2013) and Victoria (B/Brisbane/60/2008, BR/08) lineages. BR/08 was the most pathogenic in genetically modified immunocompromised mice [BALB scid and non-obese diabetic (NOD) scid strains] causing lethal infection without prior adaptation. The immunocompromised mice demonstrated prolonged virus shedding with modest induction of immune responses compared to BALB/c. Rather than severe virus burden, BR/08 virus-associated disease severity correlated with extensive virus spread and severe pulmonary pathology, stronger and persistent natural killer cell responses, and the extended induction of pro-inflammatory cytokines and chemokines. In contrast to a single-dose treatment (75 mg/kg/day), repeated doses of peramivir rescued BALB scid mice from lethal challenge with BR/08, but did not result in complete virus clearance. In summary, we have established immunocompromised murine models for influenza B virus infection that will facilitate evaluations of the efficacy of currently available and investigational anti-influenza drugs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

