Targeting primary acute myeloid leukemia with a new CXCR4 antagonist IgG1 antibody (PF-06747143)
- PMID: 28779088
- PMCID: PMC5544749
- DOI: 10.1038/s41598-017-07848-8
Targeting primary acute myeloid leukemia with a new CXCR4 antagonist IgG1 antibody (PF-06747143)
Abstract
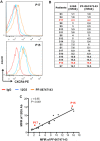
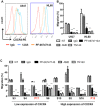
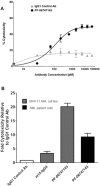
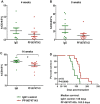
The chemokine receptor CXCR4 mediates cell anchorage in the bone marrow (BM) microenvironment and is overexpressed in 25-30% of patients with acute myeloid leukemia (AML). Here we have shown that a new CXCR4 receptor antagonist IgG1 antibody (PF-06747143) binds strongly to AML cell lines and to AML primary cells inhibiting their chemotaxis in response to CXCL12. PF-06747143 also induced cytotoxicity in AML cells via Fc-effector function. To characterize the effects of PF-06747143 on leukemia progression, we used two different patient-derived xenograft (PDX) models: Patient 17CXCR4-low and P15CXCR4-high models, characterized by relatively low and high CXCR4 expression, respectively. Weekly administration of PF-06747143 to leukemic mice significantly reduced leukemia development in both models. Secondary transplantation of BM cells from PF-06747143-treated or IgG1 control-treated animals showed that leukemic progenitors were also targeted by PF-06747143. Administration of a single dose of PF-06747143 to PDX models induced rapid malignant cell mobilization into the peripheral blood (PB). These findings support evaluation of this antibody in AML therapy, with particular appeal to patients resistant to chemotherapy and to unfit patients, unable to tolerate intensive chemotherapy.
Conflict of interest statement
Y.G., P.L., D.T., S.-H.L., T.S., V.R.F. and F.P. were employees of Pfizer, Inc. when the studies were conducted. The other authors declared no potential conflicts of interests.
Figures



References
-
- Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. - DOI - PMC - PubMed
-
- Peled A, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

