Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo
- PMID: 28779102
- PMCID: PMC5544694
- DOI: 10.1038/s41598-017-07494-0
Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo
Abstract
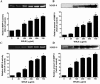
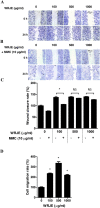
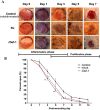
Royal jelly (RJ) has successfully been used as a remedy in wound healing. RJ has multiple effects, including antibacterial, anti-inflammatory and immunomodulatory activities, in various cell types. However, no component(s) (other than antibacterial) have been identified in RJ-accelerated wound healing. In this study, we demonstrate that keratinocytes are responsible for the elevated production of matrix metalloproteinase-9 (MMP-9) after incubation with a water extract of RJ. Furthermore, the keratinocyte migration and wound closure rates were significantly increased in the presence of RJ extract. MMP-9 production was reduced significantly following proteinase K treatment but remained stable after heat treatment, indicating that active component(s) have a proteinous character. To identify the component responsible for inducing MMP-9 production, RJ extract was fractionated using C18 RP-HPLC. In fractions exhibiting stimulatory activity, we immunochemically detected the bee-derived antibacterial peptide, defensin-1. Defensin-1 was cloned, and recombinant peptide was produced in a baculoviral expression system. Defensin-1 stimulated MMP-9 secretion from keratinocytes and increased keratinocyte migration and wound closure in vitro. In addition, defensin-1 promoted re-epithelisation and wound closure in uninfected excision wounds. These data indisputably demonstrate that defensin-1, a regular but concentration variable factor found in honey and RJ, contributes to cutaneous wound closure by enhancing keratinocyte migration and MMP-9 secretion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model.BMC Complement Altern Med. 2019 Jul 12;19(1):175. doi: 10.1186/s12906-019-2592-7. BMC Complement Altern Med. 2019. PMID: 31299973 Free PMC article.
-
The in vitro and in vivo wound-healing effects of royal jelly derived from Apis mellifera L. during blossom seasons of Castanea mollissima Bl. and Brassica napus L. in South China exhibited distinct patterns.BMC Complement Med Ther. 2020 Nov 23;20(1):357. doi: 10.1186/s12906-020-03138-5. BMC Complement Med Ther. 2020. PMID: 33225942 Free PMC article.
-
Royal jelly increases collagen production in rat skin after ovariectomy.J Med Food. 2012 Jun;15(6):568-75. doi: 10.1089/jmf.2011.1888. Epub 2012 Apr 2. J Med Food. 2012. PMID: 22468645 Free PMC article.
-
Royal Jelly: Biological Action and Health Benefits.Int J Mol Sci. 2024 May 30;25(11):6023. doi: 10.3390/ijms25116023. Int J Mol Sci. 2024. PMID: 38892209 Free PMC article. Review.
-
Royal Jelly: An ancient remedy with remarkable antibacterial properties.Microbiol Res. 2016 Nov;192:130-141. doi: 10.1016/j.micres.2016.06.007. Epub 2016 Jun 23. Microbiol Res. 2016. PMID: 27664731 Review.
Cited by
-
Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey.Sci Rep. 2018 Jun 13;8(1):9061. doi: 10.1038/s41598-018-27449-3. Sci Rep. 2018. PMID: 29899462 Free PMC article.
-
The Potential of Honeybee Products for Biomaterial Applications.Biomimetics (Basel). 2021 Jan 15;6(1):6. doi: 10.3390/biomimetics6010006. Biomimetics (Basel). 2021. PMID: 33467429 Free PMC article. Review.
-
Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering.Pharmaceutics. 2022 Mar 30;14(4):750. doi: 10.3390/pharmaceutics14040750. Pharmaceutics. 2022. PMID: 35456584 Free PMC article. Review.
-
The Antimicrobial Potential of Bacteria Isolated from Honey Samples Produced in the Apiaries Located in Pomeranian Voivodeship in Northern Poland.Int J Environ Res Public Health. 2018 Sep 14;15(9):2002. doi: 10.3390/ijerph15092002. Int J Environ Res Public Health. 2018. PMID: 30223435 Free PMC article.
-
Correlation between Honey Parameters and Wound Healing Properties: The Case of Piedmont (Italy) Samples.Curr Pharm Biotechnol. 2025;26(2):302-311. doi: 10.2174/0113892010328741240828093859. Curr Pharm Biotechnol. 2025. PMID: 39238381
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

