Epilation induces hair and skin pigmentation through an EDN3/EDNRB-dependent regenerative response of melanocyte stem cells
- PMID: 28779103
- PMCID: PMC5544680
- DOI: 10.1038/s41598-017-07683-x
Epilation induces hair and skin pigmentation through an EDN3/EDNRB-dependent regenerative response of melanocyte stem cells
Abstract
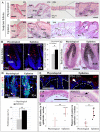
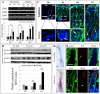
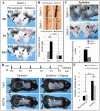
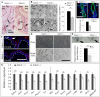
In response to various types of injury, melanocyte stem cells (McSCs) located in the bulge of hair follicles can regenerate mature melanocytes for hair and skin pigmentation. How McSCs respond to injury, however, remains largely unknown. Here we show that after epilation of mice, McSCs regenerate follicular and epidermal melanocytes, resulting in skin and hair hyperpigmentation. We further show that epilation leads to endogenous EDN3 upregulation in the dermal papilla, the secondary hair germ cells, and the epidermis. Genetic and pharmacological disruption of the EDN3 receptor EDNRB in vivo significantly blocks the effect of epilation on follicular and epidermal melanocyte regeneration as well as skin and hair hyperpigmentation. Taken together, these results indicate that epilation induces McSCs activation through EDN3/EDNRB signaling and in turn leads to skin and hair hyperpigmentation. The findings suggest that EDN/EDNRB signaling may serve as a potential therapeutic target to promote repigmentation in hypopigmentation disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling.Cell Rep. 2016 May 10;15(6):1291-302. doi: 10.1016/j.celrep.2016.04.006. Epub 2016 Apr 28. Cell Rep. 2016. PMID: 27134165 Free PMC article.
-
Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation.Pigment Cell Melanoma Res. 2011 Jun;24(3):401-10. doi: 10.1111/j.1755-148X.2011.00855.x. Epub 2011 May 5. Pigment Cell Melanoma Res. 2011. PMID: 21466661 Review.
-
SCF/c-kit signaling is required in 12-O-tetradecanoylphorbol-13-acetate-induced migration and differentiation of hair follicle melanocytes for epidermal pigmentation.Cell Tissue Res. 2015 May;360(2):333-46. doi: 10.1007/s00441-014-2101-8. Epub 2015 Mar 3. Cell Tissue Res. 2015. PMID: 25727244
-
Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration.Cell. 2011 Jun 10;145(6):941-955. doi: 10.1016/j.cell.2011.05.004. Cell. 2011. PMID: 21663796 Free PMC article.
-
Mechanisms of repigmentation induced by photobiomodulation therapy in vitiligo.Exp Dermatol. 2019 Feb;28 Suppl 1:10-14. doi: 10.1111/exd.13823. Exp Dermatol. 2019. PMID: 30698884 Review.
Cited by
-
Whole-genome resequencing reveals melanin deposition candidate genes of Luning chicken.BMC Genomics. 2024 Sep 13;25(1):858. doi: 10.1186/s12864-024-10774-5. BMC Genomics. 2024. PMID: 39271972 Free PMC article.
-
KIT ligand protects against both light-induced and genetic photoreceptor degeneration.Elife. 2020 Apr 3;9:e51698. doi: 10.7554/eLife.51698. Elife. 2020. PMID: 32242818 Free PMC article.
-
A quantitative trait locus on chromosome 2 was identified that accounts for a substantial proportion of phenotypic variance of the yellow plumage color in chicken.Poult Sci. 2020 Jun;99(6):2902-2910. doi: 10.1016/j.psj.2020.01.030. Epub 2020 Mar 5. Poult Sci. 2020. PMID: 32475423 Free PMC article.
-
Identification of lncRNAs involved in the hair follicle cycle transition of cashmere goats in response to photoperiod change.BMC Genomics. 2025 May 15;26(1):487. doi: 10.1186/s12864-025-11675-x. BMC Genomics. 2025. PMID: 40375123 Free PMC article.
-
Identification of immune-related hub genes in spinal cord injury.Eur J Med Res. 2024 Oct 4;29(1):483. doi: 10.1186/s40001-024-02075-0. Eur J Med Res. 2024. PMID: 39367463 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

