A time-resolved fluoroimmunoassay to assay the rabies virus glycoprotein: application for estimation of human rabies vaccine potency
- PMID: 28779162
- PMCID: PMC5544697
- DOI: 10.1038/s41598-017-07687-7
A time-resolved fluoroimmunoassay to assay the rabies virus glycoprotein: application for estimation of human rabies vaccine potency
Abstract
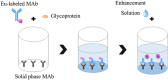
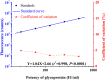
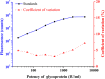
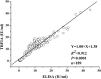
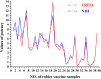
Replacement of the in vivo rabies vaccine potency test (NIH test) by in vitro methods had been discussed by several researcher including WHO expert working groups. In this paper, a time-resolved fluoroimmunoassay (TRFIA) for the assay of rabies virus glycoprotein in rabies vaccine was first established to estimate the rabies vaccine potency by using specific monoclonal antibody that only recognized the native, trimeric and immunogenic form of rabies virus glycoprotein. Potency of the rabies virus glycoprotein was assayed with satisfactory performance under optimal conditions, and the method demonstrated satisfactory results when applied in practical samples. The correlation coefficient of potency values obtained from the present TRFIA and ELISA was 0.912, and 0.903 for those from the present TRFIA and NIH test. These preliminary results confirmed that this TRFIA can replace ELISA with higher performance, and could be a promising replacement of the NIH test. Based upon these results, the present TRFIA seemed to be a convenient tool for evaluating rabies vaccine potency and its products at different stages accordingly.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Dodet, B. & Asian Rabies Expert, B. An important date in rabies history. Vaccine25, 8647–8650 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

