RNA SEQ Analysis Indicates that the AE3 Cl-/HCO3- Exchanger Contributes to Active Transport-Mediated CO2 Disposal in Heart
- PMID: 28779178
- PMCID: PMC5544674
- DOI: 10.1038/s41598-017-07585-y
RNA SEQ Analysis Indicates that the AE3 Cl-/HCO3- Exchanger Contributes to Active Transport-Mediated CO2 Disposal in Heart
Abstract
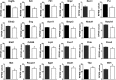
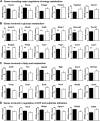
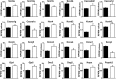
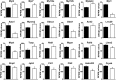
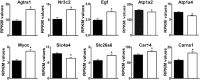
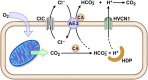
Loss of the AE3 Cl-/HCO3- exchanger (Slc4a3) in mice causes an impaired cardiac force-frequency response and heart failure under some conditions but the mechanisms are not known. To better understand the functions of AE3, we performed RNA Seq analysis of AE3-null and wild-type mouse hearts and evaluated the data with respect to three hypotheses (CO2 disposal, facilitation of Na+-loading, and recovery from an alkaline load) that have been proposed for its physiological functions. Gene Ontology and PubMatrix analyses of differentially expressed genes revealed a hypoxia response and changes in vasodilation and angiogenesis genes that strongly support the CO2 disposal hypothesis. Differential expression of energy metabolism genes, which indicated increased glucose utilization and decreased fatty acid utilization, were consistent with adaptive responses to perturbations of O2/CO2 balance in AE3-null myocytes. Given that the myocardium is an obligate aerobic tissue and consumes large amounts of O2, the data suggest that loss of AE3, which has the potential to extrude CO2 in the form of HCO3-, impairs O2/CO2 balance in cardiac myocytes. These results support a model in which the AE3 Cl-/HCO3- exchanger, coupled with parallel Cl- and H+-extrusion mechanisms and extracellular carbonic anhydrase, is responsible for active transport-mediated disposal of CO2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

