The ever-growing complexity of the mitochondrial fission machinery
- PMID: 28779209
- PMCID: PMC5765209
- DOI: 10.1007/s00018-017-2603-0
The ever-growing complexity of the mitochondrial fission machinery
Abstract
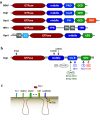
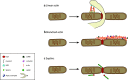
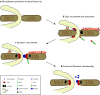
The mitochondrial network constantly changes and remodels its shape to face the cellular energy demand. In human cells, mitochondrial fusion is regulated by the large, evolutionarily conserved GTPases Mfn1 and Mfn2, which are embedded in the mitochondrial outer membrane, and by OPA1, embedded in the mitochondrial inner membrane. In contrast, the soluble dynamin-related GTPase Drp1 is recruited from the cytosol to mitochondria and is key to mitochondrial fission. A number of new players have been recently involved in Drp1-dependent mitochondrial fission, ranging from large cellular structures such as the ER and the cytoskeleton to the surprising involvement of the endocytic dynamin 2 in the terminal abscission step. Here we review the recent findings that have expanded the mechanistic model for the mitochondrial fission process in human cells and highlight open questions.
Keywords: Actin; Cytoskeleton; Drp1; Dyn2; Dynamin; ER; Mitochondrial dynamics; Mitochondrial fusion; Nucleoid; Septin; mtDNA.
Figures

Similar articles
-
New insights into the function and regulation of mitochondrial fission.Biochim Biophys Acta. 2013 May;1833(5):1256-68. doi: 10.1016/j.bbamcr.2013.02.002. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434681 Review.
-
Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function.Annu Rev Physiol. 2016;78:505-31. doi: 10.1146/annurev-physiol-021115-105011. Epub 2015 Nov 19. Annu Rev Physiol. 2016. PMID: 26667075 Review.
-
Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery.EMBO J. 2019 Apr 15;38(8):e99748. doi: 10.15252/embj.201899748. Epub 2019 Mar 6. EMBO J. 2019. PMID: 30842096 Free PMC article.
-
Regulation of mitochondrial fusion and division.Trends Cell Biol. 2007 Nov;17(11):563-9. doi: 10.1016/j.tcb.2007.08.006. Epub 2007 Oct 23. Trends Cell Biol. 2007. PMID: 17959383 Review.
-
Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics.Trends Cell Biol. 2018 Jan;28(1):67-76. doi: 10.1016/j.tcb.2017.08.011. Epub 2017 Sep 11. Trends Cell Biol. 2018. PMID: 28911913 Free PMC article. Review.
Cited by
-
COX-2 Expression in Hepatocytes Improves Mitochondrial Function after Hepatic Ischemia-Reperfusion Injury.Antioxidants (Basel). 2022 Aug 30;11(9):1724. doi: 10.3390/antiox11091724. Antioxidants (Basel). 2022. PMID: 36139798 Free PMC article.
-
Intracellular and Intercellular Mitochondrial Dynamics in Parkinson's Disease.Front Neurosci. 2019 Sep 18;13:930. doi: 10.3389/fnins.2019.00930. eCollection 2019. Front Neurosci. 2019. PMID: 31619944 Free PMC article. Review.
-
Metabolic Priming as a Tool in Redox and Mitochondrial Theragnostics.Antioxidants (Basel). 2023 May 10;12(5):1072. doi: 10.3390/antiox12051072. Antioxidants (Basel). 2023. PMID: 37237939 Free PMC article. Review.
-
Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii.Sci Rep. 2021 Jan 12;11(1):618. doi: 10.1038/s41598-020-79966-9. Sci Rep. 2021. PMID: 33436835 Free PMC article.
-
Galectin-8 drives ERK-dependent mitochondrial fragmentation, perinuclear relocation and mitophagy, with metabolic adaptations for cell proliferation.Eur J Cell Biol. 2025 Jun;104(2):151488. doi: 10.1016/j.ejcb.2025.151488. Epub 2025 Apr 4. Eur J Cell Biol. 2025. PMID: 40209344 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

