Room-temperature ionic liquids meet bio-membranes: the state-of-the-art
- PMID: 28779453
- PMCID: PMC5578922
- DOI: 10.1007/s12551-017-0279-1
Room-temperature ionic liquids meet bio-membranes: the state-of-the-art
Abstract
Room-temperature ionic liquids (RTIL) are a new class of organic salts whose melting temperature falls below the conventional limit of 100 °C. Their low vapor pressure, moreover, has made these ionic compounds the solvents of choice of the so-called green chemistry. For these and other peculiar characteristics, they are increasingly used in industrial applications. However, studies of their interaction with living organisms have highlighted mild to severe health hazards. Since their cytotoxicity shows a positive correlation with their lipophilicity, several chemical-physical studies of their interactions with biomembranes have been carried out in the last few years, aiming to identify the molecular mechanisms behind their toxicity. Cation chain length and anion nature of RTILs have seemed to affect lipophilicity and, in turn, their toxicity. However, the emerging picture raises new questions, points to the need to assess toxicity on a case-by-case basis, but also suggests a potential positive role of RTILs in pharmacology, bio-medicine and bio-nanotechnology. Here, we review this new subject of research, and comment on the future and the potential importance of this emerging field of study.
Keywords: Biomedicine; Biomembranes; Ionic liquids; Nanotechnology; Phospholipid bilayers; Toxicity.
Conflict of interest statement
Conflict of interest
Antonio Benedetto declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
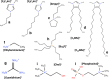
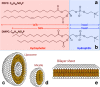
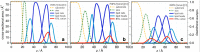
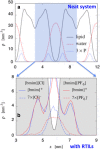

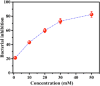
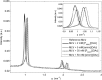
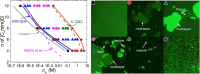
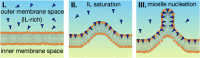

References
-
- Bee M. Principles and applications in solid state chemistry, biology and materials science. Bristol PA: Adam Hilger; 1988.
-
- Benedetto A, Ballone P. Room temperature ionic liquids meet bio-molecules: a microscopic view of structure and dynamics. ACS Sustain Chem Eng. 2016;4:392–412. doi: 10.1021/acssuschemeng.5b01385. - DOI
-
- Benedetto A, Ballone P. Room temperature ionic liquids meet bio-molecules: a microscopic view of structure and dynamics. Phi Mag. 2016;96:870–894. doi: 10.1080/14786435.2015.1119323. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

