Improving Pediatric Protein Binding Estimates: An Evaluation of α1-Acid Glycoprotein Maturation in Healthy and Infected Subjects
- PMID: 28779462
- PMCID: PMC5797516
- DOI: 10.1007/s40262-017-0576-7
Improving Pediatric Protein Binding Estimates: An Evaluation of α1-Acid Glycoprotein Maturation in Healthy and Infected Subjects
Abstract
Background: Differences in plasma protein levels observed between children and adults can alter the extent of xenobiotic binding in plasma, resulting in divergent patterns of exposure.
Objective: This study aims to quantify the ontogeny of α1-acid glycoprotein in both healthy and infected subjects.
Methods: Data pertaining to α1-acid glycoprotein from healthy subjects were compiled over 26 different publications. For subjects diagnosed or suspected of infection, α1-acid glycoprotein levels were obtained from 214 individuals acquired over three clinical investigations. The analysis evaluated the use of linear, power, exponential, log-linear, and sigmoid E max models to describe the ontogeny of α1-acid glycoprotein. Utility of the derived ontogeny equation for estimation of pediatric fraction unbound was evaluated using average-fold error and absolute average-fold error as measures of bias and precision, respectively. A comparison to fraction unbound estimates derived using a previously proposed linear equation was also instituted.
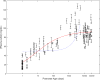
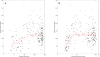
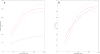
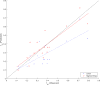
Results: The sigmoid E max model provided the comparatively best depiction of α1-acid glycoprotein ontogeny in both healthy and infected subjects. Despite median α1-acid glycoprotein levels in infected subjects being more than two-fold greater than those observed in healthy subjects, a similar ontogeny pattern was observed when levels were normalized toward adult levels. For estimation of pediatric fraction unbound, the α1-acid glycoprotein ontogeny equation derived from this work (average fold error 0.99; absolute average fold error 1.24) provided a superior predictive performance in comparison to the previous equation (average fold error 0.74; absolute average fold error 1.45).
Conclusion: The current investigation depicts a proficient modality for estimation of protein binding in pediatrics and will, therefore, aid in reducing uncertainty associated with pediatric pharmacokinetic predictions.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

