Antiepileptic drugs for chronic non-cancer pain in children and adolescents
- PMID: 28779491
- PMCID: PMC6424379
- DOI: 10.1002/14651858.CD012536.pub2
Antiepileptic drugs for chronic non-cancer pain in children and adolescents
Abstract
Background: Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization (WHO) guidelines for pharmacological treatments for children's persisting pain acknowledge that pain in children is a major public health concern of high significance in most parts of the world. While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as importantWe designed a suite of seven reviews on chronic non-cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non-steroidal anti-inflammatory drugs, opioids, and paracetamol) in order to review the evidence for children's pain utilising pharmacological interventions in children and adolescents.As the leading cause of morbidity in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain (that is pain lasting three months or longer) can occur in the paediatric population in a variety of pathophysiological classifications (nociceptive, neuropathic, or idiopathic) relating to genetic conditions, nerve damage pain, chronic musculoskeletal pain, and chronic abdominal pain, and for other unknown reasons.Antiepileptic (anticonvulsant) drugs, which were originally developed to treat convulsions in people with epilepsy, have in recent years been used to provide pain relief in adults for many chronic painful conditions and are now recommended for the treatment of chronic pain in the WHO list of essential medicines. Known side effects of antiepileptic drugs range from sweating, headache, elevated temperature, nausea, and abdominal pain to more serious effects including mental or motor function impairment.
Objectives: To assess the analgesic efficacy and adverse events of antiepileptic drugs used to treat chronic non-cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, and Embase via Ovid from inception to 6 September 2016. We also searched the reference lists of retrieved studies and reviews as well as online clinical trial registries.
Selection criteria: Randomised controlled trials, with or without blinding, by any route, treating chronic non-cancer pain in children and adolescents, comparing any antiepileptic drug with placebo or an active comparator.
Data collection and analysis: Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and number needed to treat for one additional event, using standard methods if data were available. We assessed the evidence using GRADE and created two 'Summary of findings' tables.
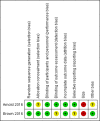
Main results: We included two studies with a total of 141 participants (aged 7 to 18 years) with chronic neuropathic pain, complex regional pain syndrome type 1 (CRPS-I), or fibromyalgia. One study investigated pregabalin versus placebo in participants with fibromyalgia (107 participants), and the other study investigated gabapentin versus amitriptyline in participants with CRPS-I or neuropathic pain (34 participants). We were unable to perform any quantitative analysis.Risk of bias for the two included studies varied, due to issues with randomisation (low to unclear risk), blinding of outcome assessors (low to unclear risk), reporting bias (low to unclear risk), the size of the study populations (high risk), and industry funding in the 'other' domain (low to unclear risk). We judged the remaining domains of sequence generation, blinding of participants and personnel, and attrition as low risk of bias. Primary outcomesOne study (gabapentin 900 mg/day versus amitriptyline 10 mg/day, 34 participants, for 6 weeks) did not report our primary outcomes (very low-quality evidence).The second study (pregabalin 75 to 450 mg/day versus placebo 75 to 450 mg/day, 107 participants, for 15 weeks) reported no significant change in pain scores for pain relief of 30% or greater between pregabalin 18/54 (33.3%), and placebo 16/51 (31.4%), P = 0.83 (very low-quality evidence). This study also reported Patient Global Impression of Change, with the percentage of participants feeling "much or very much improved" with pregabalin 53.1%, and placebo 29.5% (very low-quality evidence).We downgraded the evidence by three levels to very low for one of two reasons: due to the fact that there was no evidence to support or refute the use of the intervention, or that there were too few data and the number of events was too small to be meaningful. Secondary outcomesIn one small study, adverse events were uncommon: gabapentin 2 participants (2 adverse events); amitriptyline 1 participant (1 adverse event) (6-week trial). The second study reported a higher number of adverse events: pregabalin 38 participants (167 adverse events); placebo 34 participants (132 adverse events) (15-week trial) (very low-quality evidence).Withdrawals due to adverse events were infrequent in both studies: pregabalin (4 participants), placebo (4 participants), gabapentin (2 participants), and amitriptyline (1 participant) (very low-quality evidence).Serious adverse events were reported in both studies. One study reported only one serious adverse event (cholelithiasis and major depression resulting in hospitalisation in the pregabalin group) and the other study reported no serious adverse events (very low-quality evidence).There were few or no data for our remaining secondary outcomes (very low-quality evidence).We downgraded the evidence by three levels to very low due to too few data and the fact that the number of events was too small to be meaningful.
Authors' conclusions: This review identified only two small studies, with insufficient data for analysis.As we could undertake no meta-analysis, we were unable to comment about efficacy or harm from the use of antiepileptic drugs to treat chronic non-cancer pain in children and adolescents. Similarly, we could not comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.We know from adult randomised controlled trials that some antiepileptics, such as gabapentin and pregabalin, can be effective in certain chronic pain conditions.We found no evidence to support or refute the use of antiepileptic drugs to treat chronic non-cancer pain in children and adolescents.
Conflict of interest statement
PW: none known.
TC: none known.
LH: none known.
JC: none known; JC is a specialist paediatric pain physician and treats patients with complex pain.
RH: none known; RH is a specialist paediatric pain clinician and treat patients with chronic pain.
EK has received consulting fees for attending a research strategy meeting from Pfizer, Inc. (2015) and for protocol and research consultation from Mallinckrodt Pharmaceuticals, Inc. (2014), AstraZeneca, Inc. (2014), and Collegium Pharma (2016); EK is a specialist paediatric pain clinician and treats patients with chronic pain.
SL: none known; SL is a specialist paediatric pain clinician and treats patients with chronic pain.
NS (Sethna) has received grants from Gebauer Company for the conduct of animal studies using a topical anaesthetic (2015). NS has offered consultant expertise to Pfizer in designing a multicentre study for use of gabapentin in treatment of neuropathic pain in children (2015). NS is a co‐investigator with an ongoing multicentre Phase 3 trial of an experimental drug SMNRX [antisense oligonucleotide] for treatment of infants and children with spinal muscle atrophy (2012 to present). NS is an anaesthesiologist and manages paediatric patients with chronic pain.
NS (Schechter): none known; NS is a developmental paediatrician and treats children and adolescents with pain; NS directs the Chronic Pain Clinic at Boston Children’s Hospital and is on the faculty at Harvard Medical School.
CW: none known; CW is a paediatrician and anaesthesiologist; CW specialises in pain and treats children and adults presenting chronic pain; CW also treats patients in palliative care.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures
Update of
References
References to studies included in this review
Arnold 2016 {published data only}
Brown 2016 {published data only}
-
- Brown SC, Johnston BC, Amaria K, Watkins J, Campbell F, Pehora C, et al. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scandinavian Journal of Pain 2016;13:156‐63. - PubMed
References to studies excluded from this review
Kalita 2014 {published data only}
-
- Kalita J, Kohat AK, Misra UK, Bhoi SK. An open labeled randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. Journal of the Neurological Sciences 2014;342(1‐2):127‐32. - PubMed
Ogawa 2010 {published data only}
-
- Ogawa S, Suzuki M, Arakawa A, Yoshiyama T, Suzuki M. Long‐term efficacy and safety of pregabalin in patients with postherpetic neuralgia: results of a 52‐week, open‐label, flexible‐dose study. Masui. The Japanese Journal of Anesthesiology 2010;59(8):961‐70. - PubMed
Pramod 2011 {published data only}
-
- Pramod GV, Shambulingappa P, Shashikanth MC, Lele S. Analgesic efficacy of diazepam and placebo in patients with temporomandibular disorders: a double blind randomized clinical trial. Indian Journal of Dental Research 2011;22(3):404‐9. - PubMed
Ries 2003 {published data only}
-
- Ries M, Mengel, Kutschke G, Kim KS, Birklein F, Krummenauer F, et al. Use of gabapentin to reduce chronic neuropathic pain in Fabry disease. Journal of Inherited Metabolic Disease 2003;26(4):413‐4. - PubMed
To 2002 {published data only}
-
- To TP, Lim TC, Hill ST, Frauman AG, Cooper N, Kirsa SW, et al. Gabapentin for neuropathic pain following spinal cord injury. Spinal Cord 2002;40(6):282‐5. - PubMed
Yilmaz 2015 {published data only}
-
- Yilmaz B, Yasar E, Koroglu Omac O, Goktepe AS, Tan AK. Gabapentin vs. pregabalin for the treatment of neuropathic pain in patients with spinal cord injury: A crossover study. Turkish Journal of Physical Medicine and Rehabilitation 2015;61:1‐5.
Additional references
AMA 2013
-
- American Medical Association. Pediatric pain management. https://www.ama‐assn.org/ 2013 (accessed 25 January 2016).
AUREF 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents 2012 (accessed 16 July 2016).
Beydoun 2009
Blom 1962
-
- Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G.32883). Lancet 1962;1:839‐40. - PubMed
Caes 2016
Chong 2000
-
- Chong MS, Smith TE. Anticonvulsants for the management of pain. Pain Reviews 2000;7:129‐49.
Chong 2003
-
- Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clinical Journal of Pain 2003;19(1):59‐68. - PubMed
Cooper 2017a
Cooper 2017b
Cooper 2017c
Cooper 2017d
Dechartres 2013
Dechartres 2014
Dickenson 2007
-
- Dickenson AH, Ghandehari J. Anti‐convulsants and anti‐depressants. Handbook of Experimental Pharmacology 2007;177:145‐77. - PubMed
Dworkin 2008
Eccleston 2003
Eccleston 2017
Eroglu 2009
Errington 2008
-
- Errington AC, Stöhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage‐gated sodium channels. Molecular Pharmacology 2008;73(1):157‐69. - PubMed
FDA 2008
-
- Federal Drug Administration. Epilepsy Drugs Get Suicide Risk Warning. http://www.webmd.com/epilepsy/news/20081216/epilepsy‐drugs‐get‐suicide‐r... 2008 (Accessed 3rd August 2017).
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Guyatt 2011
Guyatt 2013a
Guyatt 2013b
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
McCleane 2000
-
- McCleane GJ. Lamotrigine in the management of neuropathic pain. Clinical Journal of Pain 2000;16:321‐6. - PubMed
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford (UK): Oxford University Press, 1998.
Moher 2009
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009
Moore 2010a
Moore 2010b
-
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. - PubMed
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010d
Moore 2010e
-
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149:173‐6. [PUBMED: 19748185] - PubMed
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] - DOI - PubMed
Moore 2011b
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014a
-
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. - PubMed
Moore 2014b
Nüesch 2010
O'Brien 2010
PedIMMPACT 2008
-
- McGrath PJ, Walco GA, Turk DC, Dworking RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT. Journal of Pain 2008;9(9):771‐83. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 2008
Ryder 2005
-
- Ryder SA, Stannard CF. Treatment of chronic pain: antidepressant, antiepileptic and antiarrhythmic drugs. Continuing Education in Anaesthesia 2005;5(1):18‐21. [DOI: 10.1093/bjaceaccp/mki003] - DOI
Stinson 2006
-
- Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self‐report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1‐2):143‐57. [DOI: 10.1016/j.pain.2006.05.006] - DOI - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Thorlund 2011
Toth 2005
-
- Toth M. The epsilon theory: a novel synthesis of the underlying molecular and electrophysiological mechanisms of primary generalized epilepsy and the possible mechanism of action of valproate. Medical Hypotheses 2005;64(2):267‐72. - PubMed
United Nations 2015
-
- United Nations. World population prospects 2015 ‐ population indicators. esa.un.org/unpd/wpp/Download/Standard/Population/ 2015 (accessed 29 February 2016).
von Baeyer 2007
WHO 2012
-
- World Health Organization. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Geneva: WHO Press, World Health Organization, 2012. [ISBN 978 92 4 154812 0] - PubMed
WHO 2015
-
- World Health Organization. 19th WHO model list of essential medicines. www.who.int/medicines/publications/essentialmedicines/EML2015_8‐May‐15.pdf 2015 (accessed 1 July 2016).
Wiffen 2013
Wiffen 2017
World Bank 2014
-
- World Bank. Data ‐ population ages 0‐14 (% of total). data.worldbank.org/indicator/SP.POP.0014.TO.ZS 2014 (accessed 29 February 2016).
Zheng 2010
-
- Zheng C, Yang K, Liu Q, Wang MY, Shen J, Valles AS, et al. The anticonvulsant drug lamotrigine blocks neuronal{alpha}4{beta}2‐nicotinic acetylcholine receptors. Journal of Pharmacology and Experimental Therapeutics 2010;335(2):401‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous