Dyrk1 inhibition improves Alzheimer's disease-like pathology
- PMID: 28779511
- PMCID: PMC5595697
- DOI: 10.1111/acel.12648
Dyrk1 inhibition improves Alzheimer's disease-like pathology
Abstract
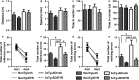
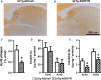
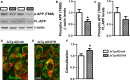
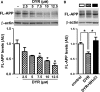
There is an urgent need for the development of new therapeutic strategies for Alzheimer's disease (AD). The dual-specificity tyrosine phosphorylation-regulated kinase-1A (Dyrk1a) is a protein kinase that phosphorylates the amyloid precursor protein (APP) and tau and thus represents a link between two key proteins involved in AD pathogenesis. Furthermore, Dyrk1a is upregulated in postmortem human brains, and high levels of Dyrk1a are associated with mental retardation. Here, we sought to determine the effects of Dyrk1 inhibition on AD-like pathology developed by 3xTg-AD mice, a widely used animal model of AD. We dosed 10-month-old 3xTg-AD and nontransgenic (NonTg) mice with a Dyrk1 inhibitor (Dyrk1-inh) or vehicle for eight weeks. During the last three weeks of treatment, we tested the mice in a battery of behavioral tests. The brains were then analyzed for the pathological markers of AD. We found that chronic Dyrk1 inhibition reversed cognitive deficits in 3xTg-AD mice. These effects were associated with a reduction in amyloid-β (Aβ) and tau pathology. Mechanistically, Dyrk1 inhibition reduced APP and insoluble tau phosphorylation. The reduction in APP phosphorylation increased its turnover and decreased Aβ levels. These results suggest that targeting Dyrk1 could represent a new viable therapeutic approach for AD.
Keywords: AD; 3xTg-AD; Alzheimer's disease; amyloid beta; plaques; tangles.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Alzheimer's A (2015) 2015 Alzheimer's disease facts and figures. Alzheimer's Dement. 11, 332–384. - PubMed
-
- Azorsa DO, Robeson RH, Frost D, Meec hoovet B, Brautigam GR, Dickey C, Beaudry C, Basu GD, Holz DR, Hernandez JA, Bisanz KM, Gwinn L, Grover A, Rogers J, Reiman EM, Hutton M, Stephan DA, Mousses S, Dunckley T (2010) High‐content siRNA screening of the kinome identifies kinases involved in Alzheimer's disease‐related tau hyperphosphorylation. BMC Genom. 11, 25. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

