Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial
- PMID: 28779963
- PMCID: PMC5594247
- DOI: 10.1016/S0140-6736(17)31145-5
Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial
Abstract
Background: Local cancer relapse risk after breast conservation surgery followed by radiotherapy has fallen sharply in many countries, and is influenced by patient age and clinicopathological factors. We hypothesise that partial-breast radiotherapy restricted to the vicinity of the original tumour in women at lower than average risk of local relapse will improve the balance of beneficial versus adverse effects compared with whole-breast radiotherapy.
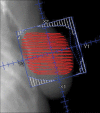
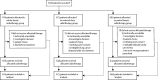
Methods: IMPORT LOW is a multicentre, randomised, controlled, phase 3, non-inferiority trial done in 30 radiotherapy centres in the UK. Women aged 50 years or older who had undergone breast-conserving surgery for unifocal invasive ductal adenocarcinoma of grade 1-3, with a tumour size of 3 cm or less (pT1-2), none to three positive axillary nodes (pN0-1), and minimum microscopic margins of non-cancerous tissue of 2 mm or more, were recruited. Patients were randomly assigned (1:1:1) to receive 40 Gy whole-breast radiotherapy (control), 36 Gy whole-breast radiotherapy and 40 Gy to the partial breast (reduced-dose group), or 40 Gy to the partial breast only (partial-breast group) in 15 daily treatment fractions. Computer-generated random permuted blocks (mixed sizes of six and nine) were used to assign patients to groups, stratifying patients by radiotherapy treatment centre. Patients and clinicians were not masked to treatment allocation. Field-in-field intensity-modulated radiotherapy was delivered using standard tangential beams that were simply reduced in length for the partial-breast group. The primary endpoint was ipsilateral local relapse (80% power to exclude a 2·5% increase [non-inferiority margin] at 5 years for each experimental group; non-inferiority was shown if the upper limit of the two-sided 95% CI for the local relapse hazard ratio [HR] was less than 2·03), analysed by intention to treat. Safety analyses were done in all patients for whom data was available (ie, a modified intention-to-treat population). This study is registered in the ISRCTN registry, number ISRCTN12852634.
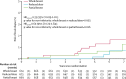
Findings: Between May 3, 2007, and Oct 5, 2010, 2018 women were recruited. Two women withdrew consent for use of their data in the analysis. 674 patients were analysed in the whole-breast radiotherapy (control) group, 673 in the reduced-dose group, and 669 in the partial-breast group. Median follow-up was 72·2 months (IQR 61·7-83·2), and 5-year estimates of local relapse cumulative incidence were 1·1% (95% CI 0·5-2·3) of patients in the control group, 0·2% (0·02-1·2) in the reduced-dose group, and 0·5% (0·2-1·4) in the partial-breast group. Estimated 5-year absolute differences in local relapse compared with the control group were -0·73% (-0·99 to 0·22) for the reduced-dose and -0·38% (-0·84 to 0·90) for the partial-breast groups. Non-inferiority can be claimed for both reduced-dose and partial-breast radiotherapy, and was confirmed by the test against the critical HR being more than 2·03 (p=0·003 for the reduced-dose group and p=0·016 for the partial-breast group, compared with the whole-breast radiotherapy group). Photographic, patient, and clinical assessments recorded similar adverse effects after reduced-dose or partial-breast radiotherapy, including two patient domains achieving statistically significantly lower adverse effects (change in breast appearance [p=0·007 for partial-breast] and breast harder or firmer [p=0·002 for reduced-dose and p<0·0001 for partial-breast]) compared with whole-breast radiotherapy.
Interpretation: We showed non-inferiority of partial-breast and reduced-dose radiotherapy compared with the standard whole-breast radiotherapy in terms of local relapse in a cohort of patients with early breast cancer, and equivalent or fewer late normal-tissue adverse effects were seen. This simple radiotherapy technique is implementable in radiotherapy centres worldwide.
Funding: Cancer Research UK.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Early-stage breast cancer: falling risks and emerging options.Lancet. 2017 Sep 9;390(10099):1010-1012. doi: 10.1016/S0140-6736(17)31599-4. Epub 2017 Aug 2. Lancet. 2017. PMID: 28779962 No abstract available.
-
Radiotherapy: Importantly, less is effective.Nat Rev Clin Oncol. 2017 Oct;14(10):585. doi: 10.1038/nrclinonc.2017.139. Epub 2017 Sep 12. Nat Rev Clin Oncol. 2017. PMID: 28895573 No abstract available.
-
Partial breast irradiation: The time is there!Breast. 2018 Apr;38:98-100. doi: 10.1016/j.breast.2017.12.014. Epub 2018 Jan 4. Breast. 2018. PMID: 29289822
-
Targeted radiotherapy for early breast cancer.Lancet. 2018 Jan 6;391(10115):26-27. doi: 10.1016/S0140-6736(17)33316-0. Lancet. 2018. PMID: 29323648 No abstract available.
-
Targeted radiotherapy for early breast cancer - Authors' reply.Lancet. 2018 Jan 6;391(10115):27-28. doi: 10.1016/S0140-6736(17)33317-2. Lancet. 2018. PMID: 29323651 No abstract available.
-
Trial supports targeted radiotherapy for early breast cancer but protocol still requires 3 weeks of daily therapy.BMJ Evid Based Med. 2018 Feb;23(1):38-39. doi: 10.1136/ebmed-2017-110849. BMJ Evid Based Med. 2018. PMID: 29367327 No abstract available.
-
Accelerated Partial Breast Radiation: Information on Dose, Volume, Fractionation, and Efficacy from Randomized Trials.Int J Radiat Oncol Biol Phys. 2020 Dec 1;108(5):1123-1128. doi: 10.1016/j.ijrobp.2020.06.064. Epub 2020 Nov 18. Int J Radiat Oncol Biol Phys. 2020. PMID: 33220220 No abstract available.
References
-
- Breast radiotherapy after breast-conserving surgery The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. CMAJ. 1998;158(suppl 3):S35–S42. - PubMed
-
- Belkacemi Y, Kaidar-Person O, Poortmans P. Patterns of practice of regional nodal irradiation in breast cancer: results of the European Organization for Research and Treatment of Cancer (EORTC) NOdal Radiotherapy (NORA) survey. Ann Oncol. 2015;26:529–535. - PubMed
-
- Dundas KL, Pogson EM, Batumalai V. Australian survey on current practices for breast radiotherapy. J Med Imaging Radiat Oncol. 2015;59:736–742. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

