Carboplatin versus two doses of cisplatin in combination with gemcitabine in the treatment of advanced non-small-cell lung cancer: Results from a British Thoracic Oncology Group randomised phase III trial
- PMID: 28780466
- PMCID: PMC5597318
- DOI: 10.1016/j.ejca.2017.05.037
Carboplatin versus two doses of cisplatin in combination with gemcitabine in the treatment of advanced non-small-cell lung cancer: Results from a British Thoracic Oncology Group randomised phase III trial
Abstract
Background: Platinum-based combination chemotherapy is standard treatment for the majority of patients with advanced non-small-cell lung cancer (NSCLC). The trial investigates the importance of the choice of platinum agent and dose of cisplatin in relation to patient outcomes.
Methods: The three-arm randomised phase III trial assigned patients with chemo-naïve stage IIIB/IV NSCLC in a 1:1:1 ratio to receive gemcitabine 1250 mg/m2 on days 1 and 8 of a 3-week cycle with cisplatin 80 mg/m2 (GC80) or cisplatin 50 mg/m2 (GC50) or carboplatin AUC6 (GCb6) for a maximum of four cycles. Primary outcome measure was survival time, aiming to test for a difference between treatment arms and also assess non-inferiority with pre-defined margin selected as hazard ratio (HR) of 1.2. Secondary outcome measures included response rate, adverse events and quality of life (QoL).
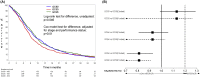
Findings: The trial recruited 1363 patients. Survival time differed significantly across the three treatment arms (p = 0.046) with GC50 worst with median 8.2 months compared to 9.5 for GC80 and 10.0 for GCb6. HRs (adjusted) for GC50 compared to GC80 was 1.13 (95% confidence interval [CI] 0.99-1.29) and for GC50 compared to GCb6 was 1.23 (95% CI: 1.08-1.41). GCb6 was significantly non-inferior to GC80 (HR = 0.93, upper limit of one-sided 95% CI 1.04). Adjusting for QoL did not change the findings. Best objective response rates were 29% (GC80), 20% (GC50) and 27% (GCb6), p < 0.007. There were more dose reductions and treatment delays in the GCb6 arm and more adverse events (60% with at least one grade 3-4 compared to 43% GC80 and 30% GC50).
Interpretation: In combination with gemcitabine, carboplatin at AUC6 is not inferior to cisplatin at 80 mg/m2 in terms of survival. Carboplatin was associated with more adverse events and not with better quality of life. Cisplatin at the lower dose of 50 mg/m2 has worse survival which is not compensated by better quality of life. CLINICALTRIALS.
Gov identifier: NCT00112710.
Eudract number: 2004-003868-30.
Cancer research uk trial identifier: CRUK/04/009.
Keywords: Carboplatin; Cisplatin; Gemcitabine; Non-small-cell lung cancer; Quality of life; Randomised phase III trial.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Quality of life comparing carboplatin with cisplatin in the treatment of non-small cell lung cancer.Eur J Cancer. 2018 Mar;91:167. doi: 10.1016/j.ejca.2017.11.019. Epub 2018 Jan 2. Eur J Cancer. 2018. PMID: 29301719 No abstract available.
References
-
- Cancer Research UK, http://www.cancerresearchuk.org/health-professional/cancer-statistics/st... [Accessed December 2016].
-
- Gettinger S., Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

