Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm
- PMID: 28780679
- PMCID: PMC5771991
- DOI: 10.1007/s00261-017-1281-6
Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm
Abstract
Radiologists play a central role in the assessment of patient response to locoregional therapies for hepatocellular carcinoma (HCC). The identification of viable tumor following treatment guides further management and potentially affects transplantation eligibility. Liver Imaging Reporting and Data Systems (LI-RADS) first introduced the concept of LR-treated in 2014, and a new treatment response algorithm is included in the 2017 update to assist radiologists in image interpretation of HCC after locoregional therapy. In addition to offering imaging criteria for viable and nonviable HCC, new concepts of nonevaluable tumors as well as tumors with equivocal viability are introduced. Existing guidelines provided by response evaluation criteria in solid tumors (RECIST) and modified RECIST address patient-level assessments and are routinely used in clinical trials but do not address the variable appearances following different locoregional therapies. The new LI-RADS treatment response algorithm addresses this gap and offers a comprehensive approach to assess treatment response for individual lesions after a variety of locoregional therapies, using either contrast-enhanced CT or MRI.
Keywords: Hepatocellular carcinoma; LI-RADS; Locoregional therapy; RECIST; Response.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
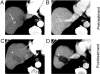
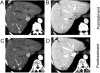
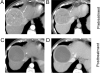
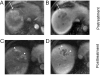
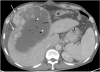
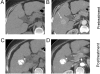
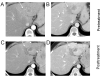
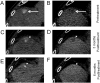


References
-
- Cheung W, et al. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12(3):233–41. - PubMed
-
- Gaba RC, et al. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol. 2016;27(4):457–73. - PubMed
-
- Benedict SH, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–101. - PubMed
-
- Halperin EC, et al. Perez and Brady's Principles and Practice of Radiation Obcology. 6. Lippincott Williams & Wilkins; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

