A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics
- PMID: 28780725
- PMCID: PMC5573753
- DOI: 10.1007/s00280-017-3387-5
A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics
Abstract
Purpose: Oral cannabinoids (i.e., dronabinol, nabilone) containing the active component of marijuana, delta(Δ)9-tetrahydrocannabinol (THC), are available for the treatment of chemotherapy-induced nausea and vomiting (CINV) in patients with cancer who have failed to adequately respond to conventional antiemetic therapy. The aim of this article is to provide an overview of the efficacy, pharmacokinetics (PK), pharmacodynamics (PD), and safety of oral cannabinoids for patients with CINV.
Methods: A PubMed search of the English-language literature available through 4 January 2017 was conducted to identify relevant articles for inclusion in the review.
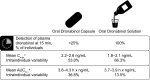
Results: Oral cannabinoids have been shown to have similar or improved efficacy compared with conventional antiemetics for the resolution of nausea and/or vomiting in patients with cancer. However, oral THC has high PK variability, with variability in oral dronabinol peak plasma concentrations (C max) estimated between 150 and 200%. A new oral dronabinol solution has decreased intraindividual variability (area under the curve) vs oral dronabinol capsules. Further, oral THC has a slower time to C max compared with THC administered intravenously (IV) or by smoking, and a lower systemic availability than IV or smoked THC. The PD profile (e.g., "high") of oral THC differs from that of IV or smoked THC in healthy individuals. Oral cannabinoids are associated with greater incidence of adverse effects compared with conventional antiemetic therapy or placebo (e.g., dizziness, hypotension, and dysphoria or depression).
Conclusions: A new formulation of oral cannabinoids (i.e., dronabinol oral solution) minimized the PK/PD variability currently observed with capsule formulations.
Keywords: Cannabinoids; Chemotherapy-induced nausea and vomiting; Dronabinol; Pharmacokinetics; THC.
Conflict of interest statement
M.E.B. reports no conflicts of interest. Technical editorial and medical writing assistance was provided under the direction of M.E.B. by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this support was provided by Insys Development Company, Chandler, AZ.
Figures

References
-
- Escobar Y, Cajaraville G, Virizuela JA, Alvarez R, Munoz A, Olariaga O, Tames MJ, Muros B, Lecumberri MJ, Feliu J, Martinez P, Adansa JC, Martinez MJ, Lopez R, Blasco A, Gascon P, Calvo V, Luna P, Montalar J, Del BP, Tornamira MV. Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer. 2015;23(9):2833–2840. doi: 10.1007/s00520-015-2809-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

