The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey
- PMID: 28780729
- PMCID: PMC5569657
- DOI: 10.1007/s00277-017-3082-y
The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey
Abstract
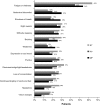
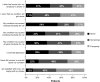
Myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) are myeloproliferative neoplasms (MPNs) associated with high disease burden, reduced quality of life (QOL), and shortened survival. To assess how MPNs affect patients, we conducted a global MPN Landmark survey. This online survey of patients with MPNs and physicians was conducted in Australia, Canada, Germany, Japan, Italy, and the United Kingdom. The survey measured MPN-related symptoms and the impact of MPNs on QOL and the ability to work as well as disease-management strategies. Overall, 219 physicians and 699 patients (MF, n = 174; PV, n = 223; ET, n = 302) completed the survey; 90% of patients experienced MPN-related symptoms. The most frequent and severe symptom was fatigue. Most patients experienced a reduction in QOL, including those with low symptom burden or low-risk scores. A substantial proportion of patients reported impairment at work and in overall activity. Interestingly, physician feedback and blood counts were the most important indicators of treatment success among patients, with improvements in symptoms and QOL being less important. Regarding disease management, our study revealed a lack of alignment between physician and patient perceptions relating to communication and disease management, with patients often having different treatment goals than physicians. Overall, our study suggested that therapies that reduce symptom burden and improve QOL in patients with MPNs are crucial in minimizing disease impact on patient daily lives. Additionally, our findings showed a need for improved patient-physician communication, standardized monitoring of symptoms, and agreement on treatment goals.
Keywords: (4–6): MPN; Activities of daily living; Quality of life; Symptom burden; Work productivity.
Conflict of interest statement
Figures



References
-
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County study, 1976-1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(SICI)1096-8652(199905)61:1<10::AID-AJH3>3.0.CO;2-I. - DOI - PubMed
-
- Johansson P, Kutti J, Andréasson B, Safai-Kutti S, Vilén L, Wedel H, Ridell B. Trends in the incidence of chronic Philadelphia chromosome negative (Ph−) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983-99. J Intern Med. 2004;256:161–165. doi: 10.1111/j.1365-2796.2004.01357.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

