Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment
- PMID: 28781050
- PMCID: PMC5568465
- DOI: 10.1016/j.cub.2017.06.084
Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment
Abstract
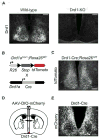
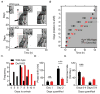
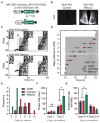
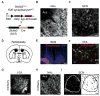
Dopamine (DA) neurotransmission controls behaviors important for survival, including voluntary movement, reward processing, and detection of salient events, such as food or mate availability. Dopaminergic tone also influences circadian physiology and behavior. Although the evolutionary significance of this input is appreciated, its precise neurophysiological architecture remains unknown. Here, we identify a novel, direct connection between the DA neurons of the ventral tegmental area (VTA) and the suprachiasmatic nucleus (SCN). We demonstrate that D1 dopamine receptor (Drd1) signaling within the SCN is necessary for properly timed resynchronization of activity rhythms to phase-shifted light:dark cycles and that elevation of DA tone through selective activation of VTA DA neurons accelerates photoentrainment. Our findings demonstrate a previously unappreciated role for direct DA input to the master circadian clock and highlight the importance of an evolutionarily significant relationship between the circadian system and the neuromodulatory circuits that govern motivational behaviors.
Keywords: D1 dopamine receptor; SCN; circadian entrainment; dopamine; dopaminergic neurons; hypothalamic circuitry; jet lag; photoentrainment; suprachiasmatic nucleus; ventral tegmental area.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


References
-
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

