Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial
- PMID: 28781108
- PMCID: PMC5831666
- DOI: 10.1016/S0140-6736(17)31585-4
Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has neuroprotective effects in preclinical models of Parkinson's disease. We investigated whether these effects would be apparent in a clinical trial.
Methods: In this single-centre, randomised, double-blind, placebo-controlled trial, patients with moderate Parkinson's disease were randomly assigned (1:1) to receive subcutaneous injections of exenatide 2 mg or placebo once weekly for 48 weeks in addition to their regular medication, followed by a 12-week washout period. Eligible patients were aged 25-75 years, had idiopathic Parkinson's disease as measured by Queen Square Brain Bank criteria, were on dopaminergic treatment with wearing-off effects, and were at Hoehn and Yahr stage 2·5 or less when on treatment. Randomisation was by web-based randomisation with a two strata block design according to disease severity. Patients and investigators were masked to treatment allocation. The primary outcome was the adjusted difference in the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) motor subscale (part 3) in the practically defined off-medication state at 60 weeks. All efficacy analyses were based on a modified intention-to-treat principle, which included all patients who completed any post-randomisation follow-up assessments. The study is registered at ClinicalTrials.gov (NCT01971242) and is completed.
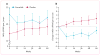
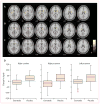
Findings: Between June 18, 2014, and March 13, 2015, 62 patients were enrolled and randomly assigned, 32 to exenatide and 30 to placebo. Our primary analysis included 31 patients in the exenatide group and 29 patients in the placebo group. At 60 weeks, off-medication scores on part 3 of the MDS-UPDRS had improved by 1·0 points (95% CI -2·6 to 0·7) in the exenatide group and worsened by 2·1 points (-0·6 to 4·8) in the placebo group, an adjusted mean difference of -3·5 points (-6·7 to -0·3; p=0·0318). Injection site reactions and gastrointestinal symptoms were common adverse events in both groups. Six serious adverse events occurred in the exenatide group and two in the placebo group, although none in either group were judged to be related to the study interventions.
Interpretation: Exenatide had positive effects on practically defined off-medication motor scores in Parkinson's disease, which were sustained beyond the period of exposure. Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Exenatide represents a major new avenue for investigation in Parkinson's disease, and effects on everyday symptoms should be examined in longer-term trials.
Funding: Michael J Fox Foundation for Parkinson's Research.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
AJL reports grants from the Frances and Renee Hock Fund, consulting fees from Britannia Pharmaceuticals (Genus) and BIAL Portela, and honoraria from Profile Pharma, Teva, Lundbeck, BIAL, Roche, Britannia, UCB, Nordiclnfu Care, NeuroDerm, and Decision Resources. TTW has received honoraria from Britannia Pharmaceuticals. PL has received honoraria from Medtronic and St Jude Medical. NHG is a named inventor on a National Institutes of Health patent describing the use of GLP-1 receptor agonists in neurodegenerative disorders. All rights to this patent belong solely to the US Government. TF has received honoraria from Profile Pharma, BIAL, AbbVie, Genus, Medtronic, and St Jude Medical. All other authors declare no competing interests.
Figures
Comment in
-
Insulin signalling: new target for Parkinson's treatments?Lancet. 2017 Oct 7;390(10103):1628-1630. doi: 10.1016/S0140-6736(17)32101-3. Epub 2017 Aug 3. Lancet. 2017. PMID: 28781109 No abstract available.
-
Repurposed drugs for use in Parkinson's disease.J Neurol. 2018 Mar;265(3):728-730. doi: 10.1007/s00415-018-8772-4. J Neurol. 2018. PMID: 29423610 No abstract available.
References
-
- Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2014;11:25–40. - PubMed
-
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–69. - PubMed
-
- Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–05. - PubMed
-
- Rampersaud N, Harkavyi A, Giordano G, Lever R, Whitton J, Whitton PS. Exendin-4 reverses biochemical and behavioral deficits in a pre-motor rodent model of Parkinson’s disease with combined noradrenergic and serotonergic lesions. Neuropeptides. 2012;46:183–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

